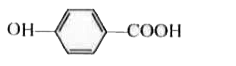
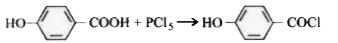A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALDEHYDES, KETONES AND CARBOXYLIC ACIDS
MTG-WBJEE|Exercise WB JEE PREVIOUS YEARS QUESTIONS(CATEGORY 1: SINGLE OPTION CORRECT TYPE)|7 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
MTG-WBJEE|Exercise WB JEE PREVIOUS YEARS QUESTIONS(CATEGORY 2: SINGLE OPTION CORRECT TYPE)|2 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
MTG-WBJEE|Exercise WB JEE WORKOUT (CATEGORY 2: SINGLE OPTION CORRECT TYPE)|15 VideosALCOHOLS AND ETHERS
MTG-WBJEE|Exercise WB JEE Previous years questions (CATEGORY 3: One or More than One Option Correct Type (2 Marks))|1 VideosALIPHATIC AMINES
MTG-WBJEE|Exercise WB JEE Previous Years Questions ( CATEGORY 3 : One or More than One Option Corred Type (2 Marks) )|1 Videos
Similar Questions
Explore conceptually related problems
MTG-WBJEE-ALDEHYDES, KETONES AND CARBOXYLIC ACIDS-WB JEE WORKOUT(CATEGORY 3: ONE OR MORE THAN ONE OPTION CORRECT TYPE)
- Which one of the following is produced when acetone is saturated with ...
Text Solution
|
- Which of the following reactions are Claisen condensation?
Text Solution
|
- Which of the following reactions will give cinnamic acid?
Text Solution
|
- Which of the following will give aldchyde only?
Text Solution
|
- Select the correct combination of reactions.
Text Solution
|
- Base-catalysed aldol condensation occurs with
Text Solution
|
- Which of the following are examples of aldol condensation?
Text Solution
|
- The products obtained in the following reaction are underset(COOH)ov...
Text Solution
|
- Which of the following compounds will give red precipitate when heated...
Text Solution
|
- PhCH(2)CH(2)COOH overset(Ag(2)O)to PhCH(2)CH(2)COOAg overset(Br(2))to ...
Text Solution
|
- When one mole of an organic compound X is reacted with an excess of PC...
Text Solution
|
- Which of the following reagents will reduce a carboxylic acid to a 1^(...
Text Solution
|
- Which of the following method(s) would be useful for preparing ketones...
Text Solution
|
- The product (A) of following reaction CH(3)COOH underset(2. Delta)ov...
Text Solution
|
- Which of the following reagents are used in the conversion of
Text Solution
|