Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
XII BOARDS PREVIOUS YEAR-XII BOARDS-SET I
- A thin spherical shell of radius R has charge Q spread uniformly over ...
Text Solution
|
- Three identical capacitors C(1) , C(2) and C(3) of capacitance 6 mu F...
Text Solution
|
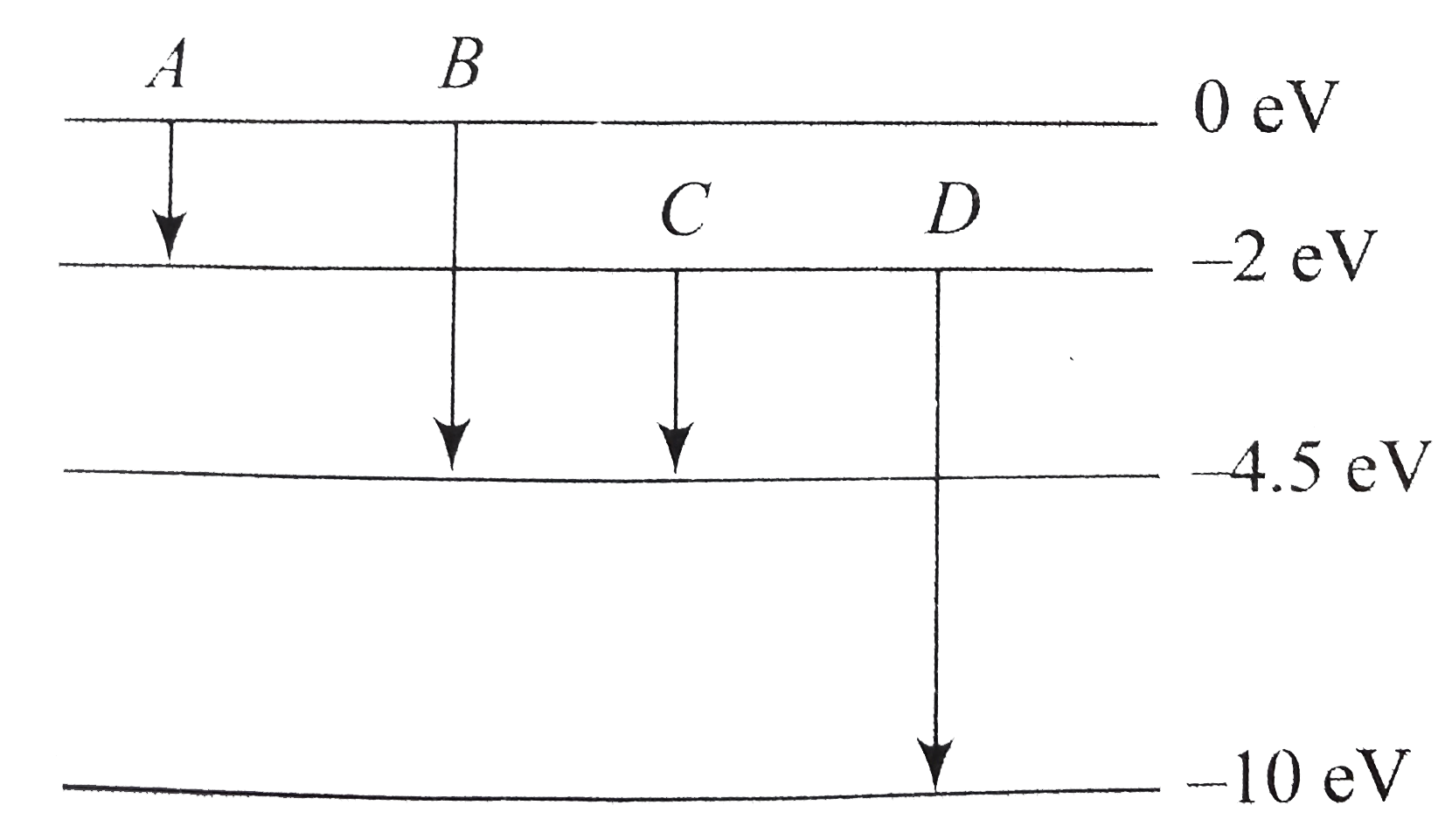
- The energy levels of an atom are as shown in figure . Which one of tho...
Text Solution
|
- A proton and alpha particle are accelerated through the same accelerat...
Text Solution
|
- In a singleslit diffraction experiment, when a tiny circular obstacle ...
Text Solution
|
- (a) Define self inductance. Write its S.I. units (b) Derive and expr...
Text Solution
|
- The figure shows experimental set up of a meter bridge. When the two ...
Text Solution
|
- Derive the expression for force per unit length between two long strai...
Text Solution
|
- Explain the principle and working of a cyclotron with the help of a sc...
Text Solution
|
- Three light rays red (R), green (G) and blue (B) are incident on a rig...
Text Solution
|
- (a) Deriver and expression for the average power consumed in a series ...
Text Solution
|
- (a) Derive the relationship between the peak and the rms value of curr...
Text Solution
|
- (i) Draw a circuit diagram to study the input and output characteristi...
Text Solution
|
- The given input A, B are fed to a 2-input NAND gate. Draw the output w...
Text Solution
|
- Trace the ays of light showing the formation of an image due to a poin...
Text Solution
|
- Draw the labelled ray diagram for the formation of image by a compound...
Text Solution
|
- When electrons drift in a metal from lower to higher potential, does i...
Text Solution
|
- At a place, the horizontal component of earth's magnetic field is B an...
Text Solution
|
- Show on a graph, the variation of resistivity with temperature for a t...
Text Solution
|
- Why should electrostatic field be zero inside a conductor ?
Text Solution
|