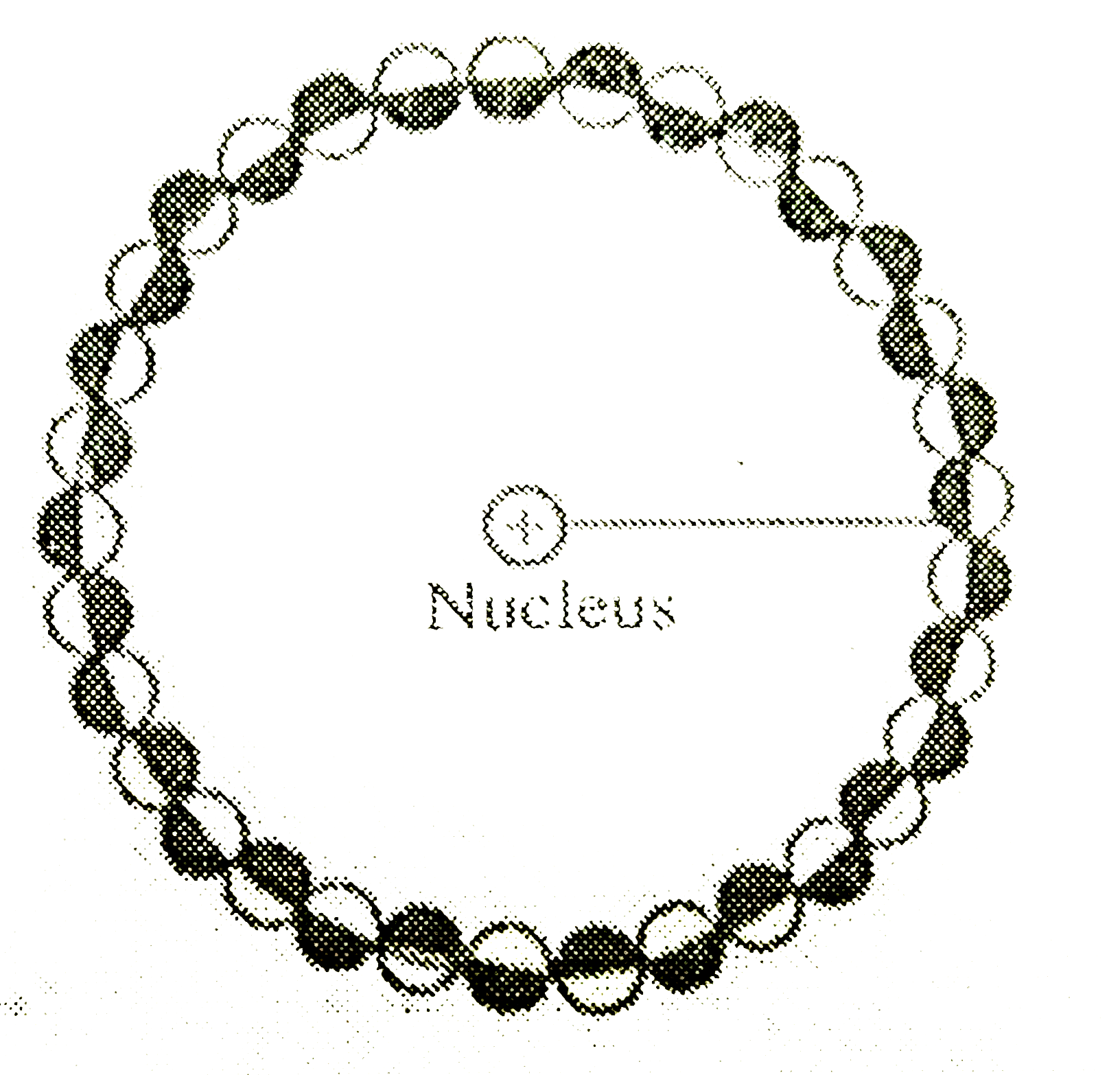
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
XII BOARDS PREVIOUS YEAR-XII BOARDS-SET-I
- Use the mirror equation to deduct that : (a) an object between f and...
Text Solution
|
- The figure below shows the V-I characteristic of a semiconductor diode...
Text Solution
|
- (a) Using de Broglie's hypothesis, explain with the help of a suitable...
Text Solution
|
- You are given a circuit below. Write its truth table. Hence, identify ...
Text Solution
|
- A compound microscope uses an objective lens of focal length 4 cm and ...
Text Solution
|
- (i) A giant refracting telescope at an observatory has an objective le...
Text Solution
|
- A resistor R(1) consumes electrical power P(1) when connected to an e...
Text Solution
|
- Prove that an ideal capacitor in an a.c. circuit does not dissipate po...
Text Solution
|
- Using Ampere's circuital law, obtaiin the expression for themagnetic f...
Text Solution
|
- State the working of a.c. generator with the help of a labelled diagra...
Text Solution
|
- (a) Show that an a.c. circuit containing a pure inductor, the voltage ...
Text Solution
|
- Two charges of magnitude -2Q and +Q are located at points (a,0) and (...
Text Solution
|
- How is mutual inductance of a pair of coils affected when (i) separati...
Text Solution
|
- The graph shown in the figure represents a plot of current versus volt...
Text Solution
|
- Two identical cells each of emf epsilon, having negligible internal re...
Text Solution
|
- The motion of copper plate is damped when it is allowed to oscillate b...
Text Solution
|
- Define the activity of a radionuclide. Write its SI unit. Give a plot ...
Text Solution
|
- Welders wear special goggles or face masks with glass windows to potec...
Text Solution
|
- Write the expression for the de-Broglie wavelength associated with a c...
Text Solution
|
- Draw typical output characteristics of an n-p-n transistor in CE confi...
Text Solution
|