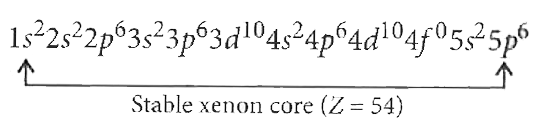A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMISTRY OF METALS
MTG-WBJEE|Exercise WB JEE PREVIOUS YEARS QUESTIONS(CATEGORY 2 : Single Option Correct Type)|1 VideosCHEMISTRY OF METALS
MTG-WBJEE|Exercise WB JEE PREVIOUS YEARS QUESTIONS(CATEGORY 3 : One or More Option Correct Type)|5 VideosCHEMISTRY OF METALS
MTG-WBJEE|Exercise WB WORKOUT (CATEGORY 3 : One or More Option Correct Type)|10 VideosCHEMISTRY OF CARBON COMPOUNDS
MTG-WBJEE|Exercise WB JEE PREVIOUS YEARS QUESTIONS (CATEGORY 3 : ONE OR MORE THAN ONE OPTION CORRECT TYPE )|3 VideosCHEMISTRY OF NON-METALLIC ELEMENTS AND THEIR COMPOUNDS
MTG-WBJEE|Exercise WB JEE PREVIOUS YEARS QUESTIONS(CATEGORY 3 : One or More Option Correct Type)|1 Videos
Similar Questions
Explore conceptually related problems
MTG-WBJEE-CHEMISTRY OF METALS-WB JEE PREVIOUS YEARS QUESTIONS(CATEGORY 1 : Single Option Correct Type)
- The ore chromite is
Text Solution
|
- Metal ion responsible for the Minamata disease is
Text Solution
|
- Consider the following salts: NaCl, HgCl(2), Hg(2)Cl(2), CuCl(2), CuCl...
Text Solution
|
- Extraction of gold (Au) involves the formation of complex ions 'X' and...
Text Solution
|
- The atomic number of cerium (Ce) is 58. The correct electronic config...
Text Solution
|
- Which of the following statements regarding lanthanides is false?
Text Solution
|
- The metal which can be used to obtain metallic Cu from aqueous CuSO(4)...
Text Solution
|
- The correct bhasicity order of the folloiwing lanthanide ions is
Text Solution
|
- Out of the following outer electronic configurations of atoms, the hig...
Text Solution
|
- Which of the following is least thermally stable ?
Text Solution
|
- In the Bacyer's process, the leaching of alumina is done by using
Text Solution
|
- Addition of sodium thiousulphate solution to a solution of silver nitr...
Text Solution
|
- [X]+dil, H(2)SO(4) to [y] colourless suffocating gas [y]+K(2)Cr(2)O(7)...
Text Solution
|
- A copper coin was electroplated with Zn and then heated at high temper...
Text Solution
|