Similar Questions
Explore conceptually related problems
Recommended Questions
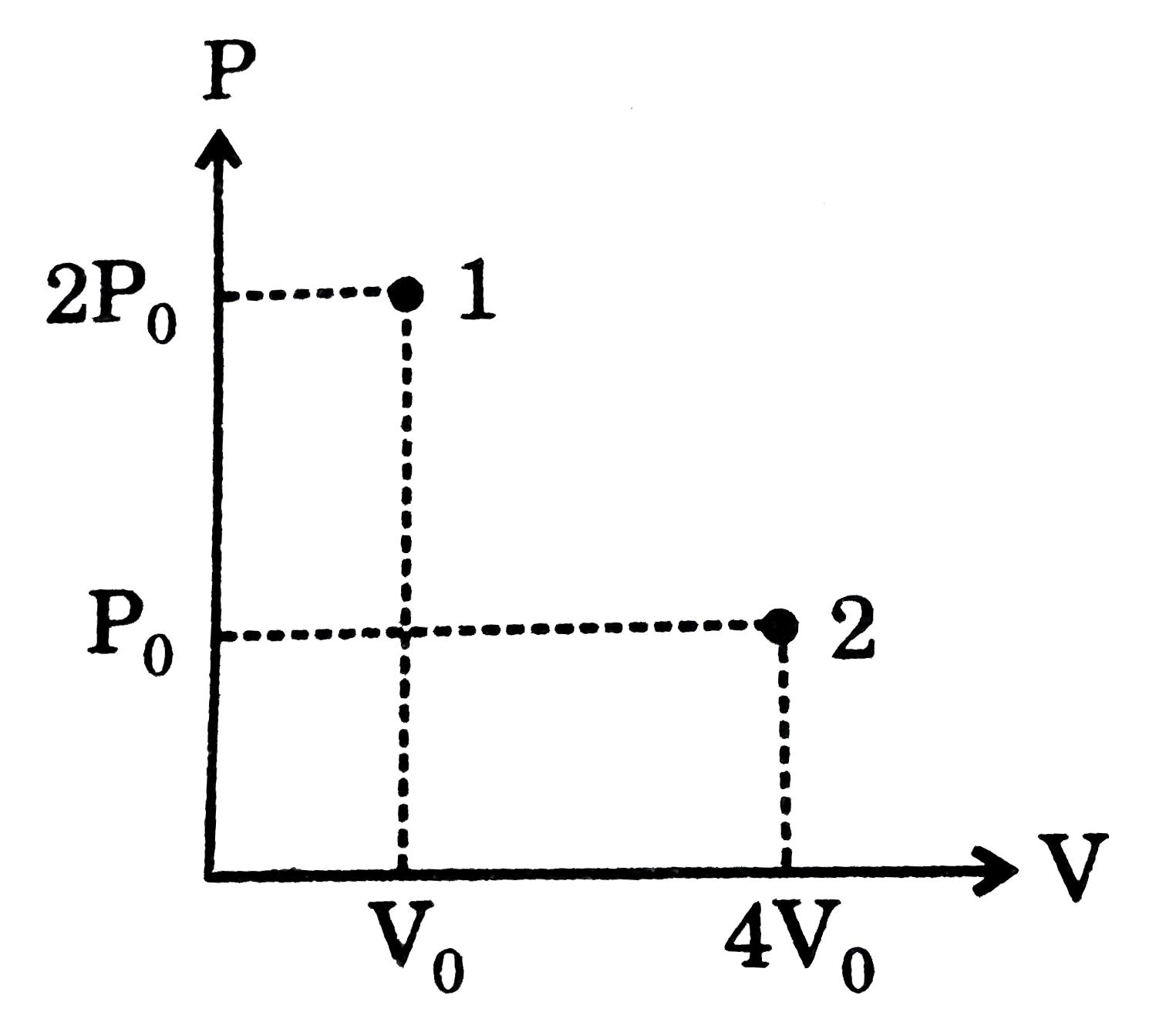
- A liquid which is confined inside an adiabatic piston is suddenly take...
Text Solution
|
- A liquid confined inside an adiabatic is suddenly taken from state 1 t...
Text Solution
|
- A liquid which is confined inside an adiabatic piston is suddently tak...
Text Solution
|
- A liquid which is confined inside an adiabatic piston is suddenly take...
Text Solution
|
- If the plot 1 rarr 2 represents an infinite stage adiabatic process an...
Text Solution
|
- A liquid confined inside an adiabatic container is suddenly taken fro...
Text Solution
|
- is the process of change of the liquid state into vapour state.
Text Solution
|
- This process of changing of solid state into liquid state is called
Text Solution
|
- process of change of state from gaseous state to liquid state is calle...
Text Solution
|