Similar Questions
Explore conceptually related problems
Recommended Questions
- Consider the change in oxidation state of Bromine corredponding to dif...
Text Solution
|
- Consider the change in oxidation state of Bromine corredponding to dif...
Text Solution
|
- Consider the change in oxidation state of bromine corresponding to dif...
Text Solution
|
- Consider the change in oxidation state of bromide corresponding to dif...
Text Solution
|
- Consider the change in oxidation state of Bromine corresponding to dif...
Text Solution
|
- Consider the change in oxidation state of Bromine corresponding to di...
Text Solution
|
- Consider the change in the oxidation states of bromine depending on th...
Text Solution
|
- Consider the change in oxidation state of Bromine corresponding to dif...
Text Solution
|
- नीचे दिए गए यौगिक का नाम है
Text Solution
|
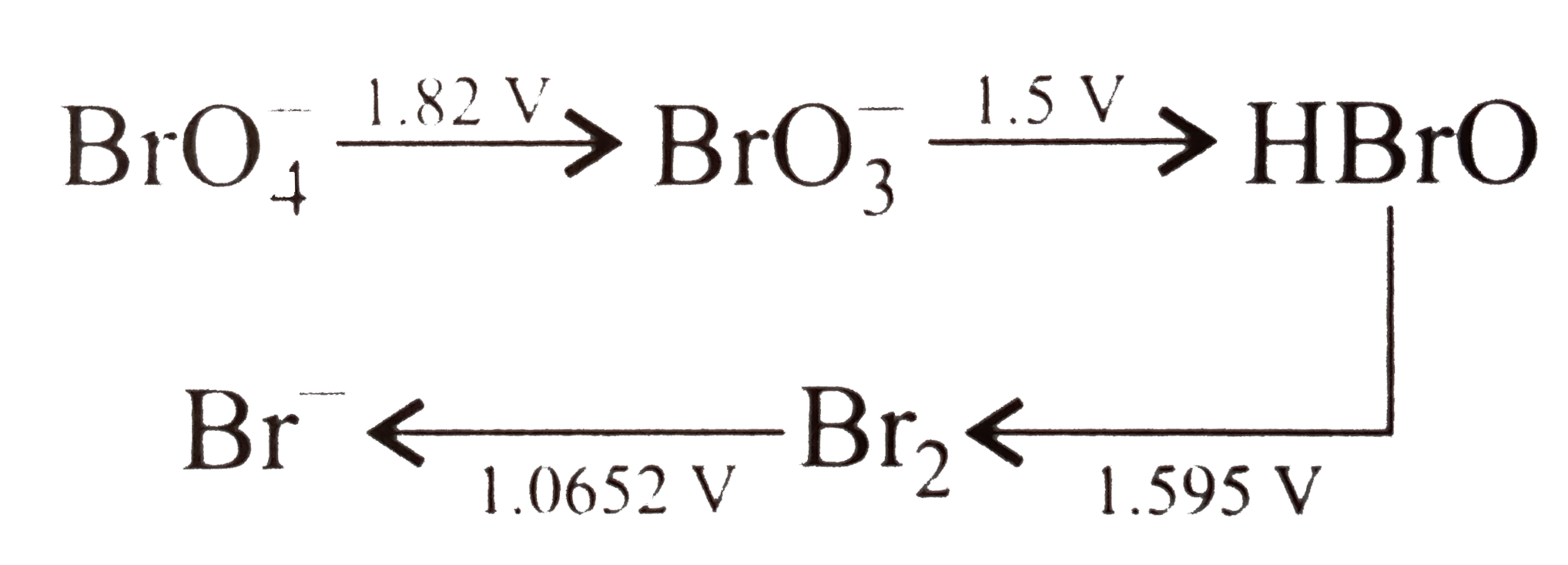 .
.