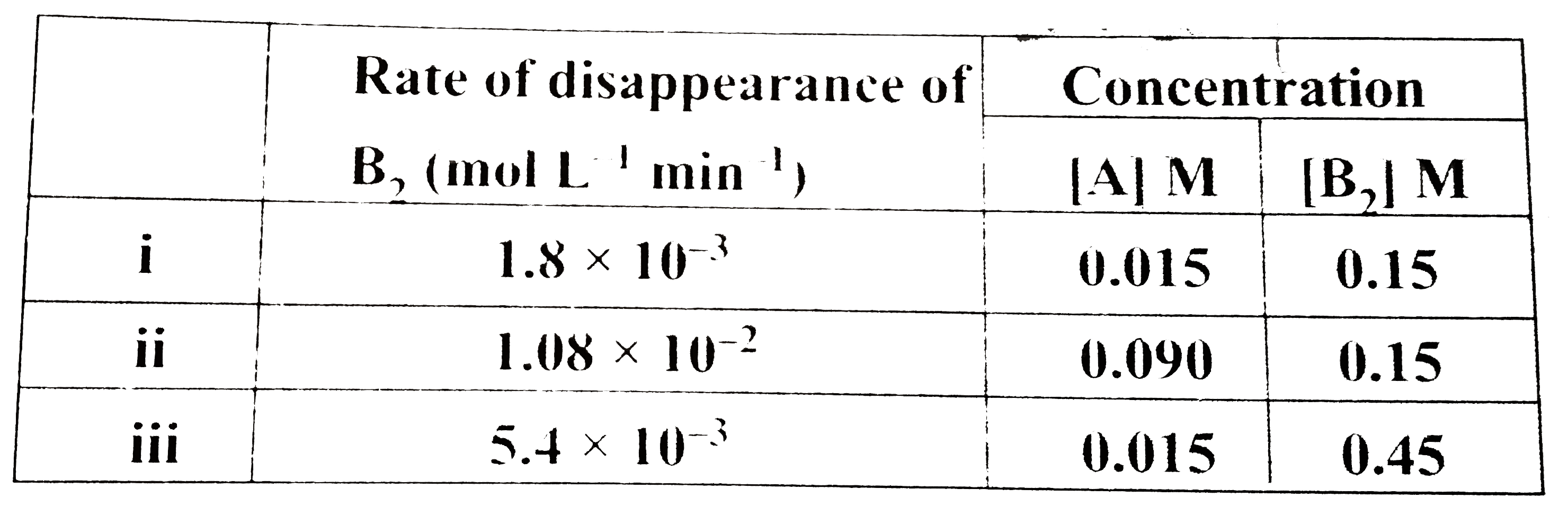Similar Questions
Explore conceptually related problems
Recommended Questions
- form the gaseous reaction 2A + B(2) rarr 2AB, the following rate dat...
Text Solution
|
- form the gaseous reaction 2A + B(2) rarr 2AB, the following rate dat...
Text Solution
|
- The following data were obtained at 300K for the reaction 2A + B to C+...
Text Solution
|
- The following initial rate data were obtained at 300K for the reaction...
Text Solution
|
- The experimental data for the reaction 2A + B(2) to 2AB is The rate eq...
Text Solution
|
- किसी अभिक्रिया, 2A+B2rarr2AB के लिए प्राप्त निम्न आंकड़ों से ज्ञात कीजि...
Text Solution
|
- For a gaseous reaction 2A+B(2)to2AB, the following rate data were obta...
Text Solution
|
- For the reaction: 2A+BrarrA(2)B the rate k[A][B]^(2) " with "k=2.0xx10...
Text Solution
|
- The experiment data for the reaction 2A + B(2) rarr 2AB is |{:("Expe...
Text Solution
|