Similar Questions
Explore conceptually related problems
Recommended Questions
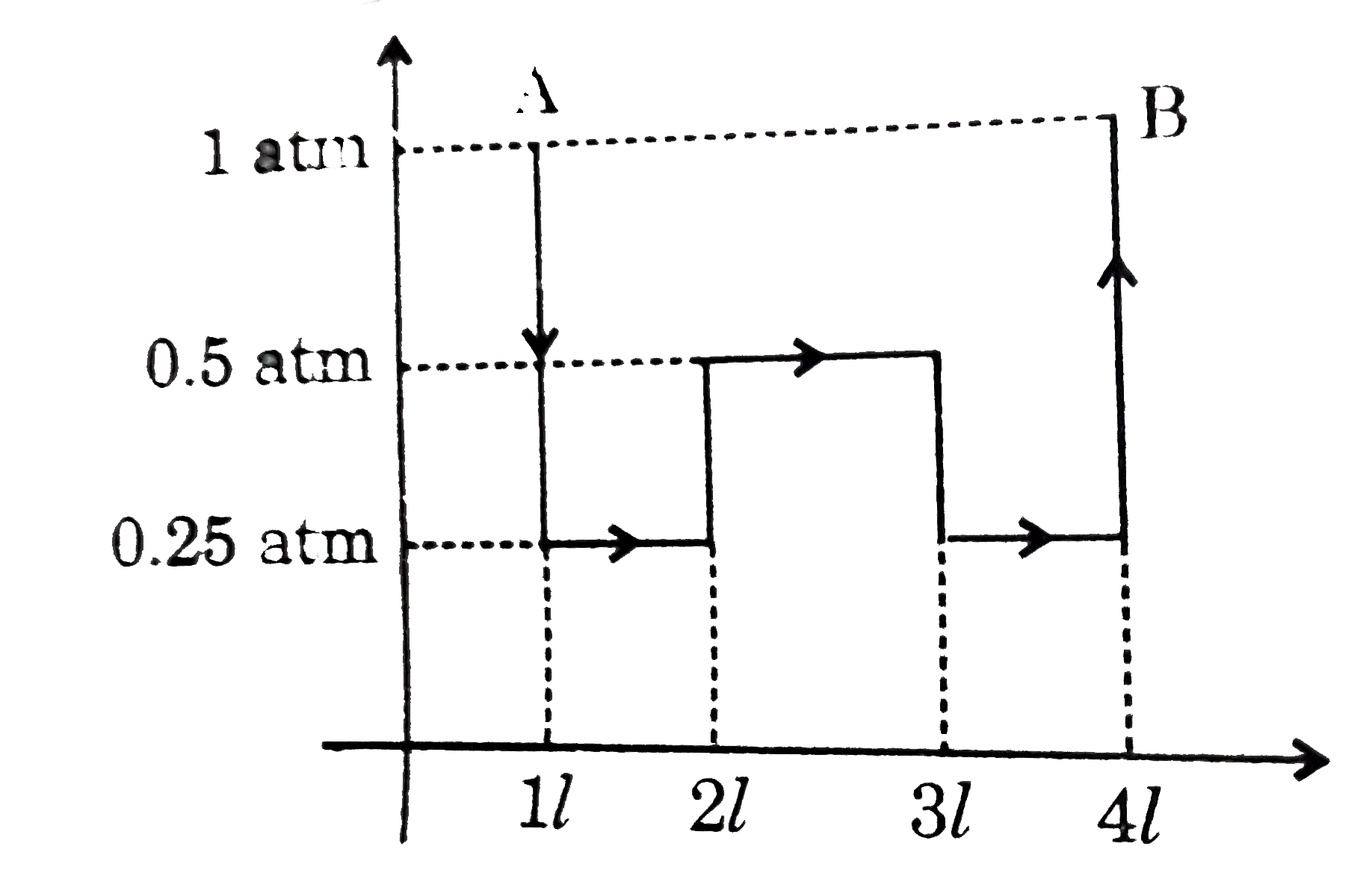
- An ideal gas which has gamma = 4//3 is taken from A to B according to ...
Text Solution
|
- For the given ideal gas equation PV=nRT , answer the following questio...
Text Solution
|
- In which of these diagrams. Density of an ideal gas remains constant?
Text Solution
|
- The change in enthalpy for an isobaric gaseous reaction (for an ideal ...
Text Solution
|
- One mole of an ideal and monoatomic gas is expanded reversibly and iso...
Text Solution
|
- An ideal gas which has gamma = 4//3 is taken from A to B according to ...
Text Solution
|
- One mole of an ideal gas undergoes a cyclic change ABCD. From the give...
Text Solution
|
- Statement-1 : There is no change in enthalpy of an ideal gas during co...
Text Solution
|
- Statement-1 : There is no change in enthalpy of an ideal gas during co...
Text Solution
|