Text Solution
Verified by Experts
Topper's Solved these Questions
ATOMS
U-LIKE SERIES|Exercise LONG ANSWER QUESTIONS - II|4 VideosATOMS
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST (SECTION A( MULTIPLE CHOICE QUESTIONS))|5 VideosATOMS
U-LIKE SERIES|Exercise SHORT ANSWER QUESTIONS|30 VideosALTERNATING CURRENT
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST (SECTION -C)|2 VideosCBSE EXAMINATION PAPER 2020
U-LIKE SERIES|Exercise SECTION D|12 Videos
Similar Questions
Explore conceptually related problems
U-LIKE SERIES-ATOMS-LONG ANSWER QUESTIONS - I
- Draw a schematic arrangement of the Geiger-Marsden experiment. How did...
Text Solution
|
- Draw a schematic arrangement of Geiger-Marsden experiment showing the ...
Text Solution
|
- In a Geiger Marsden experiment, calculate the distance of closest appr...
Text Solution
|
- Using Bohr's postulates , obtain the expreesion for the total energy ...
Text Solution
|
- (a)Using Bohr's postulates, derive the expression for the total energy...
Text Solution
|
- Explain the origin of spectral lines of hydrogen using Bohr's theory.
Text Solution
|
- The ground state energy of hydrogen atom is -13.6 eV. (i) What is th...
Text Solution
|
- (i) State Bohr's quantisation condition for defining stationary orbits...
Text Solution
|
- The ground state energy of hydrogen atom is - 13.6 eV. If an electron ...
Text Solution
|
- (a) State Bohr's postulate to define stable orbits in hydrogen atom. H...
Text Solution
|
- The value of ground state energy of hydrogen atom is - 13.6 eV. (a) ...
Text Solution
|
- The energy of the electron in the ground state of hydrogen is - 13.6 e...
Text Solution
|
- The electron in a given Bohr orbit E(n) = - 1.54 eV. Calculate (i) i...
Text Solution
|
- The energy levels of a hypothetical atom are shown in Fig.12.13. Which...
Text Solution
|
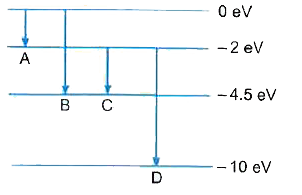
- The energy level diagram of an element is given below. Identify, by do...
Text Solution
|
- The fig.12.15 showes energy level diagram of hydrogen atom . (a) F...
Text Solution
|
- State the limitation of Bohr's theory.
Text Solution
|
- (a) Using Bohr's second postulate of quantisation of orbital angular m...
Text Solution
|