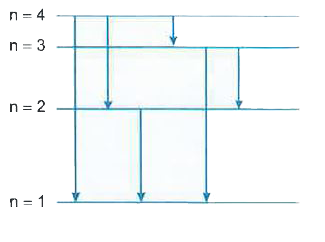
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
EXAMINATION PAPER 2020 (SOLVED)
U-LIKE SERIES|Exercise SECTION C|8 VideosEXAMINATION PAPER 2020 (SOLVED)
U-LIKE SERIES|Exercise SECTION D|6 VideosEXAMINATION PAPER 2020 (SOLVED)
U-LIKE SERIES|Exercise SECTION D|6 VideosELECTROSTATIC POTENTIAL AND CAPACITANCE
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST|1 VideosMAGNETISM AND MATTER
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST (Section D)|1 Videos
Similar Questions
Explore conceptually related problems
U-LIKE SERIES-EXAMINATION PAPER 2020 (SOLVED)-SECTION B
- Two cells of emf E(1) and E(2)(E(1)gtE(2)) are connected as shown in t...
Text Solution
|
- Two identical bars, one of paramagnetic material and other of diamagne...
Text Solution
|
- Two coplanar and concentric coils 1 and 2 have respectively the number...
Text Solution
|
- How does an oscillating charge radiate an electromagnetic wave ? Give ...
Text Solution
|
- (a) Explain briefly the fact that electromagnetic waves carry energy. ...
Text Solution
|
- A converging lens of focal length f(1) is placed coaxially in contact ...
Text Solution
|
- How is the resolving power of a compound microscope affected if (a) wa...
Text Solution
|
- Define the terms (a) threshold frequency, and (b) stopping potential. ...
Text Solution
|
- A hydrogen atom is in its third excited state. (a) How many spectral...
Text Solution
|