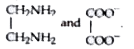Text Solution
Verified by Experts
Topper's Solved these Questions
COORDINATION COMPOUNDS
U-LIKE SERIES|Exercise CASE BASED/SOURCE-BASED INTEGRATED QUESTION|15 VideosCOORDINATION COMPOUNDS
U-LIKE SERIES|Exercise MULTIPLE CHOICE QUESTIONS|20 VideosCOORDINATION COMPOUNDS
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST|7 VideosCHEMISTRY IN EVERYDAY LIFE
U-LIKE SERIES|Exercise LONG ANSWER QUESTIONS - I|21 VideosELECTROCHEMISTRY
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST (SECTION D)|1 Videos
Similar Questions
Explore conceptually related problems
U-LIKE SERIES-COORDINATION COMPOUNDS-NCERT TEXTBOOK EXERCISES
- FeSO(4) solution mixed with (NH(4))(2)SO(4) solution in 1 : 1 molar ra...
Text Solution
|
- What is meant by unidentate, didentate and ambidentate ligands ? Give ...
Text Solution
|
- Specify the oxidation numbers of the metals in the following coordinat...
Text Solution
|
- Specify the oxidation numbers of the metals in the following coordinat...
Text Solution
|
- Specify the oxidation numbers of the metals in the following coordinat...
Text Solution
|
- Specify the oxidation numbers of the metals in the following coordinat...
Text Solution
|
- Specify the oxidation numbers of the metals in the following coordinat...
Text Solution
|
- Using IUPAC norms write the formulas for the following: Tetrahydroxo...
Text Solution
|
- Using IUPAC norms write the formulas for the following: Potassium te...
Text Solution
|
- Using IUPAC norms write the formulas for the following: Diamminedich...
Text Solution
|
- Using IUPAC norms write the formulas for the following: Potassium te...
Text Solution
|
- Using IUPAC norms write the formulas for the following: Pentaamminen...
Text Solution
|
- Using IUPAC norms write the formulas for the following: Hexaammineco...
Text Solution
|
- Using IUPAC norms write the formulas for the following: Potassium tr...
Text Solution
|
- Using IUPAC norms write the formulas for the following: Hexaamminepl...
Text Solution
|
- Using IUPAC norms write the formulas for the following: Tetrabromido...
Text Solution
|
- Using IUPAC norms write the formulas for the following: Pentaamminen...
Text Solution
|
- Using IUPAC norms, write the systematic names of the following: [Co(...
Text Solution
|
- Using IUPAC norms, write the systematic names of the following: [Pt(...
Text Solution
|
- Using IUPAC norms, write the systematic names of the following: [Ti(...
Text Solution
|