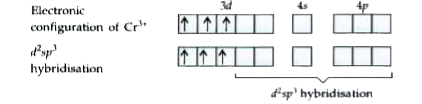
Text Solution
Verified by Experts
Topper's Solved these Questions
COORDINATION COMPOUNDS
U-LIKE SERIES|Exercise CASE BASED/SOURCE-BASED INTEGRATED QUESTION|15 VideosCOORDINATION COMPOUNDS
U-LIKE SERIES|Exercise MULTIPLE CHOICE QUESTIONS|20 VideosCOORDINATION COMPOUNDS
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST|7 VideosCHEMISTRY IN EVERYDAY LIFE
U-LIKE SERIES|Exercise LONG ANSWER QUESTIONS - I|21 VideosELECTROCHEMISTRY
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST (SECTION D)|1 Videos
Similar Questions
Explore conceptually related problems
U-LIKE SERIES-COORDINATION COMPOUNDS-NCERT TEXTBOOK EXERCISES
- Discuss the nature of bonding in the following coordination entities o...
Text Solution
|
- Discuss the nature of bonding in the following coordination entities o...
Text Solution
|
- Discuss the nature of bonding in the following coordination entities o...
Text Solution
|
- What is spectrochemical series ? Explain the difference between a weak...
Text Solution
|
- What is crystal field splitting energy ? How does the magnitude of Del...
Text Solution
|
- [Cr(NH(3))(6)]^(3+) is paramagnetic while [Ni(CN)(4)]^(2-) is diamagne...
Text Solution
|
- A solution of [Ni(H(2)O)(6)]^(2+) is green but a solution of [Ni(CN)(4...
Text Solution
|
- [Fe(CN)(6)]^(4-) and [Fe(H(2)O)(6)]^(2+) are of different colours in d...
Text Solution
|
- Give the oxidation state, d-orbital occupation and coordination number...
Text Solution
|
- Write down the IUPAC name for each of the following complexes and indi...
Text Solution
|
- Write down the IUPAC name for each of the following complexes and indi...
Text Solution
|
- Write down the IUPAC name for each of the following complexes and indi...
Text Solution
|
- Write down the IUPAC name for each of the following complexes and indi...
Text Solution
|
- Write down the IUPAC name for each of the following complexes and indi...
Text Solution
|
- What is meant by the chelate effect ? Give an example.
Text Solution
|
- How many ions are produced from the complex Co(NH(3))(6)Cl(2) in solut...
Text Solution
|
- Amongst the following ions, which one has the highest magnetic moment ...
Text Solution
|
- The oxidation number of cobalt in K[Co(CO)(4)] is
Text Solution
|
- Amongst the following, the most stable complex is :
Text Solution
|
- What will be the correct order for the wavelengths of absorption in th...
Text Solution
|