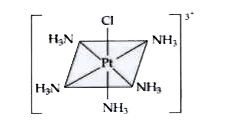
Text Solution
Verified by Experts
Topper's Solved these Questions
COORDINATION COMPOUNDS
U-LIKE SERIES|Exercise LONG ANSWER QUESTIONS-I (3 marks each)|85 VideosCOORDINATION COMPOUNDS
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST|7 VideosCOORDINATION COMPOUNDS
U-LIKE SERIES|Exercise VERY SHORT ANSWER QUESTIONS (1 mark each)|69 VideosCHEMISTRY IN EVERYDAY LIFE
U-LIKE SERIES|Exercise LONG ANSWER QUESTIONS - I|21 VideosELECTROCHEMISTRY
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST (SECTION D)|1 Videos
Similar Questions
Explore conceptually related problems
U-LIKE SERIES-COORDINATION COMPOUNDS-SHORT ANSWER QUESTIONS (2 marks each)
- CuSO(4).5H(2)O is blue in colour while CuSO(4) is colourless, why ?
Text Solution
|
- Name the type of isomerism when ambidentate ligands are attached to co...
Text Solution
|
- Write the IUPAC name and draw the structure of coordination entities o...
Text Solution
|
- Using valence bond theory, predict the shape and magnetism (paramagnet...
Text Solution
|
- Why are low spin tetrahedral complexes not formed ?
Text Solution
|
- Using the valence bond approach, deduce the shape and magnetic charact...
Text Solution
|
- How is the magnitude of Delta(0) affected by (i) nature of ligand and ...
Text Solution
|
- Using the valence bond approach, deduce the shape and magnetic charact...
Text Solution
|
- Using valence bond approach, deduce the shape and magnetic behaviour o...
Text Solution
|
- Using the valence bond approach, predict the shape and magnetic charac...
Text Solution
|
- Using the valence bond approach, predict the shape and magnetic behavi...
Text Solution
|
- Using the valence bond approach, deduce the shape and magnetic behavio...
Text Solution
|
- Deduce the shape and magnetic behaviour of the complex ion [Co(NH(3))(...
Text Solution
|
- Among [Ag(NH(3))(2)]Cl, [Ni(CN)(4))^(2-) and [CuCl(4)]^(2-) which ha...
Text Solution
|
- Among [Ag(NH(3))(2)]Cl, [Ni(CN)(4))^(2-) and [CuCl(4)]^(2-) which re...
Text Solution
|
- Square planar complexes with coordination number 4 exhibit geometrical...
Text Solution
|
- Using valence bond approach, predict the shape and magnetic character ...
Text Solution
|
- Explain the following : [Co(NH(3))(6)]^(3+) is diamagnetic, whereas ...
Text Solution
|
- Explain the following : [Fe(H(2)O)(6)]^(3+) is more paramagnetic tha...
Text Solution
|
- Write the state of hybridisation and the oxidation state of the centra...
Text Solution
|