Text Solution
Verified by Experts
Topper's Solved these Questions
COORDINATION COMPOUNDS
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST|7 VideosCOORDINATION COMPOUNDS
U-LIKE SERIES|Exercise SHORT ANSWER QUESTIONS (2 marks each)|54 VideosCHEMISTRY IN EVERYDAY LIFE
U-LIKE SERIES|Exercise LONG ANSWER QUESTIONS - I|21 VideosELECTROCHEMISTRY
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST (SECTION D)|1 Videos
Similar Questions
Explore conceptually related problems
U-LIKE SERIES-COORDINATION COMPOUNDS-LONG ANSWER QUESTIONS-I (3 marks each)
- Draw the geometrical isomers of complex [Pt(en)(2)Cl(2)]^(2+) .
Text Solution
|
- On the basis of crystal field theory, write the electronic configurati...
Text Solution
|
- Write the hybridisation type and magnetic behaviour of the complex [Ni...
Text Solution
|
- Write the IUPAC name of the complex [Cr(NH(3)) 4Cl(2)]Cl.
Text Solution
|
- What type of isomerism is exhibited by the complex [Co(en)(3)]^(3+) ? ...
Text Solution
|
- Why is [NiCl(4)]^(2-) paramagnetic but [Ni(CO)(4)] is diamagnetic ? ...
Text Solution
|
- Write the IUPAC name of the complex [Cr(NH(3))(4)Cl(2)]^(+).
Text Solution
|
- What type of isomerism does it exhibit ?
Text Solution
|
- Draw the structures of these geometrical isomers.
Text Solution
|
- What is meant by crystal field splitting energy ? On the basis of crys...
Text Solution
|
- What is meant by crystal field splitting energy ? On the basis of crys...
Text Solution
|
- Name the following coordination entities and draw the structures of th...
Text Solution
|
- Name the following coordination entities and draw the structures of th...
Text Solution
|
- Name the following coordination entities and draw the structures of th...
Text Solution
|
- For the complex [Fe(en)(2)Cl(2)]Cl, identify the following : Oxidati...
Text Solution
|
- For the complex [Fe(en)(2)Cl(2)]Cl, identify the following : Hybrid ...
Text Solution
|
- For the complex [Fe(en)(2)Cl(2)]Cl, identify the following : Magneti...
Text Solution
|
- For the complex [Fe(en)(2)Cl(2)]Cl, identify the following : Magneti...
Text Solution
|
- For the complex [Fe(en)(2)Cl(2)]Cl, identify the following : Magneti...
Text Solution
|
- For the complex [Fe(en)(2)Cl(2)]Cl, identify the following : Name of...
Text Solution
|
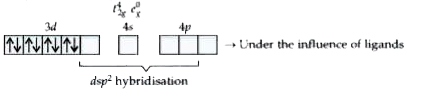 `to` Under the influence of ligands
`to` Under the influence of ligands