Text Solution
Verified by Experts
Topper's Solved these Questions
ACIDS, BASES AND SALTS
U-LIKE SERIES|Exercise CASE BASED / SOURCE-BASED INTEGRTED QUESTIONS|12 VideosACIDS, BASES AND SALTS
U-LIKE SERIES|Exercise MULTIPLE CHOICE QUESTIONS (ONE MARK EACH)|24 VideosACIDS, BASES AND SALTS
U-LIKE SERIES|Exercise LONG ANSWER QUESTIONS|12 VideosCARBON AND ITS COMPOUNDS
U-LIKE SERIES|Exercise LONG ANSWER QUESTIONS|4 Videos
Similar Questions
Explore conceptually related problems
U-LIKE SERIES-ACIDS, BASES AND SALTS-NCERT EXERCISES
- A solution turns red litmus blue, its pH is likely to be
Text Solution
|
- A solution reacts with crushed egg-shells to give a gas that turns lim...
Text Solution
|
- 10 mL of a solution of NaOH is found to be completely neutralised by 8...
Text Solution
|
- Which one of the following types of medicines is used for treating ind...
Text Solution
|
- Write word equations and then balanced equations for the reaction taki...
Text Solution
|
- Write word equations and then balanced equations for the reaction taki...
Text Solution
|
- Write word equations and then balanced equations for the reaction taki...
Text Solution
|
- Write word equations and then balanced equations for the reaction taki...
Text Solution
|
- Compounds such as alcohols and glucose also contain hydrogen but are n...
Text Solution
|
- Why does distilled water not conduct electricity, whereas rain water d...
Text Solution
|
- Why do acids not show acidic behaviour in the absence of water ?
Text Solution
|
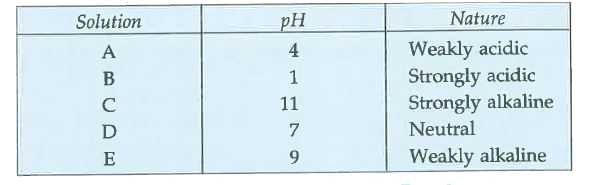
- Five solutions A, B, C, D and E when tested with universal indicator s...
Text Solution
|
- Equal lengths of magnesium ribbons are taken in test tubes A and B. Hy...
Text Solution
|
- Fresh milk has a pH of 6. How do you think the pH will change as it tu...
Text Solution
|
- A milkman adds a very small amount of baking soda to fresh milk. (a...
Text Solution
|
- A milkman adds a very small amount of baking soda to fresh milk. Wh...
Text Solution
|
- Plaster of Paris should be stored in a moisture-proof container. Expla...
Text Solution
|
- What is a neutralisation reaction ? Give two examples.
Text Solution
|
- Give two important uses of washing soda and baking soda.
Text Solution
|