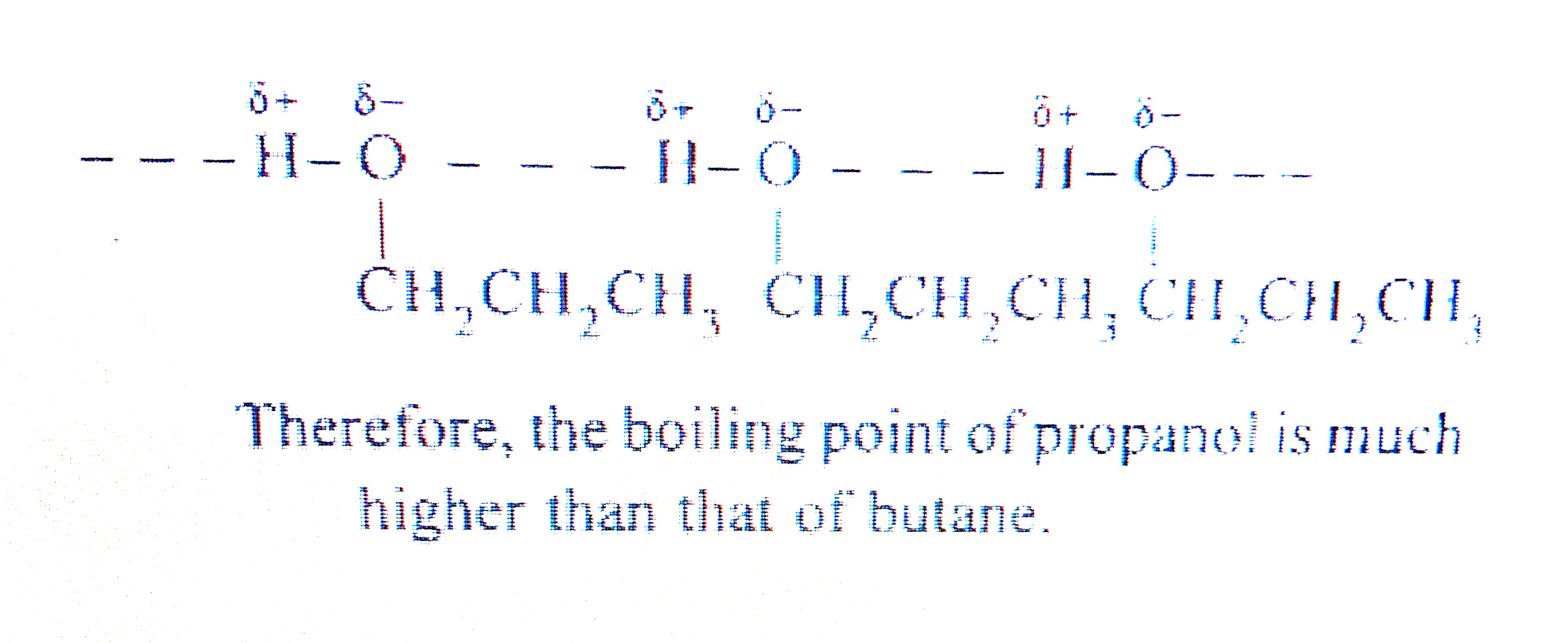Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Explain why propanol has a higher boiling point than hydrocarbon butan...
Text Solution
|
- Explain why propanol has a higher boiling point than hydrocarbon butan...
Text Solution
|
- समझाइए कि प्रोपेनॉल का क्वथनांक, हाइड्रोकार्बन ब्यूटेन से अधिक क्यों ह...
Text Solution
|
- स्पष्ट कीजिए की प्रोपेनोल का क्वथनांक ब्यूटेन से अधिक क्यों होता है?
Text Solution
|
- Explain why propanol has higher boiling point than that of the hydroca...
Text Solution
|
- समझाइए कि प्रोपेनॉल का क्वथनांक, हाइड्रोकार्बन ब्यूटेन से अधिक क्यों ह...
Text Solution
|
- Explain why propanol has a higher boiling point than hydrocarbon butan...
Text Solution
|
- प्रोपेनॉल का क्वथनांक संगत ऐल्केन की अपेक्षा उच्च होता है क्यों?
Text Solution
|
- Explain why propanol has higher boiling point than that of the hydroca...
Text Solution
|