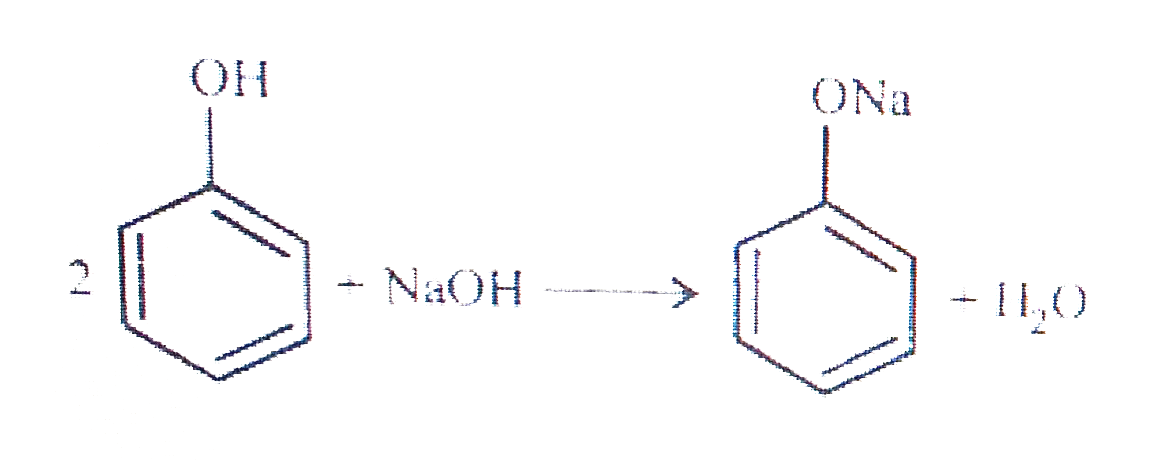Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
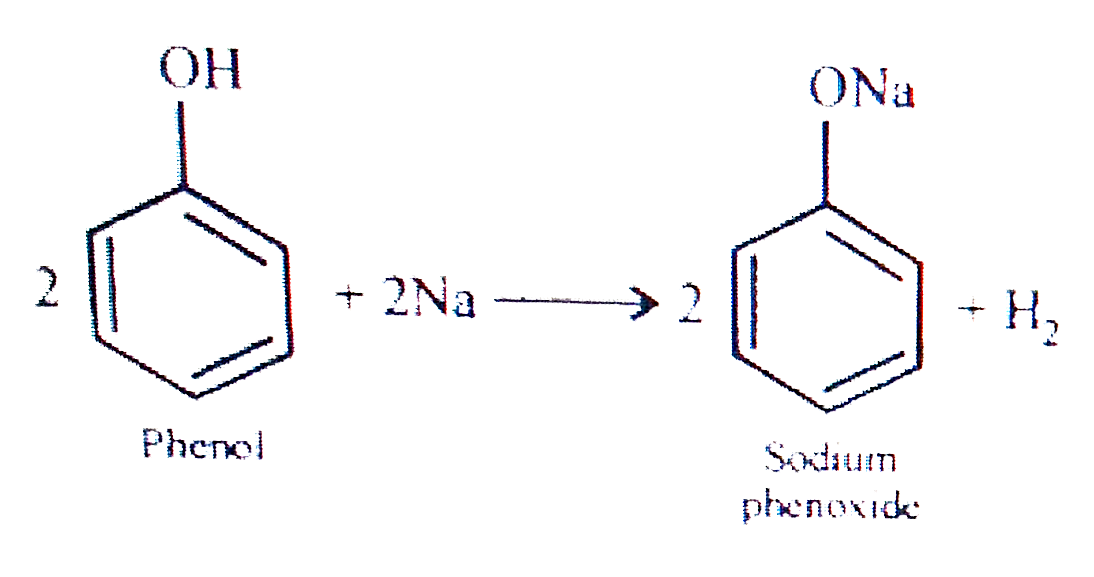
- Give two reactions that show the acidic nature of phenol. Compare the ...
Text Solution
|
- Give two reactions that show the acidic nature of phennol. Compare the...
Text Solution
|
- ऐसी दो अभिक्रियाए दीजिए जिनसे फीनॉल की अम्लीय प्रकृति प्रदर्शित होती ह...
Text Solution
|
- ऐसी दो अभिक्रियाएँ लिखिए, जिनमें फीनॉल की अम्लीय प्रकृति प्रदर्शित होत...
Text Solution
|
- ऐसी दो अभिक्रियाएँ दीजिए जिनसे फीनॉल की अम्लीय प्रकृति प्रदर्शित होती ...
Text Solution
|
- फिनॉल की अम्लीय प्रकृति को प्रदर्शित करने के लिए दो अभिक्रियाएँ दीजिए।...
Text Solution
|
- फीनॉल की अम्लता की तुलन एथेनॉल की अम्लता से कीजिए।
Text Solution
|
- ऐसी दो अभिक्रियाएँ दीजिए जिनसे फिनॉल की अम्लीय प्रकृति प्रदर्शित होती ...
Text Solution
|
- Give two reactions that show the acidic nature of phenol. Compare its ...
Text Solution
|