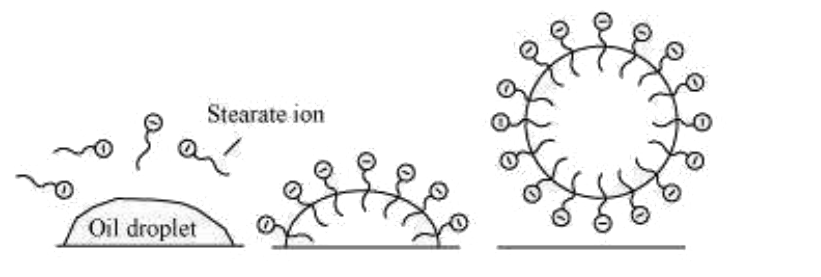Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT ENGLISH-CHEMISTRY IN EVERYDAY LIFE-Exercise
- Low level of noradrenaline is the cause of depression. What type of dr...
Text Solution
|
- What is meant by the term ‘broad spectrum antibiotics’ ? Explain.
Text Solution
|
- How do antiseptics differ from disinfectants ? Give one example of eac...
Text Solution
|
- Why are cimetidine and ranitidine better antacids than sodium hydrogen...
Text Solution
|
- Name a substance which can be used as an antiseptic as well as disinfe...
Text Solution
|
- What are the main constituents of dettol ?
Text Solution
|
- What is tincture of iodine ? What is its use ?
Text Solution
|
- What are food preservatives ?
Text Solution
|
- Why is use of aspartame limited to cold foods and drinks ?
Text Solution
|
- What are artificial sweetening agents ? Give two examples.
Text Solution
|
- Name the sweetening agent used in the preparation of sweets for a diab...
Text Solution
|
- What is the problem faced while using alitame as artificial sweetner ?
Text Solution
|
- How are synthetic detergents better than soaps ?
Text Solution
|
- Explain the following terms with suitable examples (i) cationic dete...
Text Solution
|
- What is the biodegradable polymer?Give an example of a biodegradable a...
Text Solution
|
- Why do soaps not work in hard water ?
Text Solution
|
- What are soaps and synthetic detergents ? What are the advantages of s...
Text Solution
|
- Explain the cleansing action of soaps.
Text Solution
|
- If water contains dissolved calcium hydrogencarbonate, out of soaps an...
Text Solution
|
- Label the hydrophilic and hydrophobic parts in the following compounds...
Text Solution
|