Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
NCERT ENGLISH-PERIODIC CLASSIFICATION OF ELEMENTS-Exercise
- Use Mendeléev’s Periodic Table to predict the formulae for the oxides ...
Text Solution
|
- Besides gallium, which other elements have since been discovered that ...
Text Solution
|
- What were the criteria used by Mendeléev in creating his Periodic Tabl...
Text Solution
|
- Why do you think the noble gases are placed in a separate group?
Text Solution
|
- How could the Modern Periodic Table remove various anomalies of Mendel...
Text Solution
|
- Name two elements you would expect to show chemical reactions similar ...
Text Solution
|
- Name (a) three elements that have a single electron in their outermo...
Text Solution
|
- (a) Lithium, sodium, potassium are all metals that react with water to...
Text Solution
|
- In the Modern Periodic Table, which are the metals among the first ten...
Text Solution
|
- By considering their position in the Periodic Table, which one of the ...
Text Solution
|
- Which of the following statements is not a correct statement about the...
Text Solution
|
- Element X forms a chloride with the formula XCl(2), which is a solid w...
Text Solution
|
- Which element has (a) two shells, both of which are completely filled...
Text Solution
|
- (a) What property do all elements in the same column of the Periodic T...
Text Solution
|
- An atom has electronic configuration 2, 8, 7. (a) What is the atomic n...
Text Solution
|
- The position of three elements A, B and C in the Periodic Table are sh...
Text Solution
|
- Nitrogen (atomic number 7) and phosphorus (atomic number 15) belong to...
Text Solution
|
- How does the electronic configuration of an atom relate to its positio...
Text Solution
|
- In the Modern Periodic Table, calcium (atomic number 20) is surrounded...
Text Solution
|
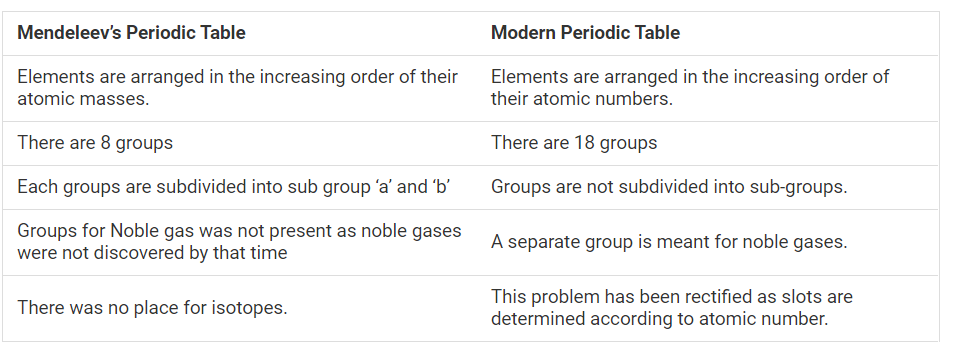
- Compare and contrast the arrangement of elements in Mendeléev’s Period...
Text Solution
|