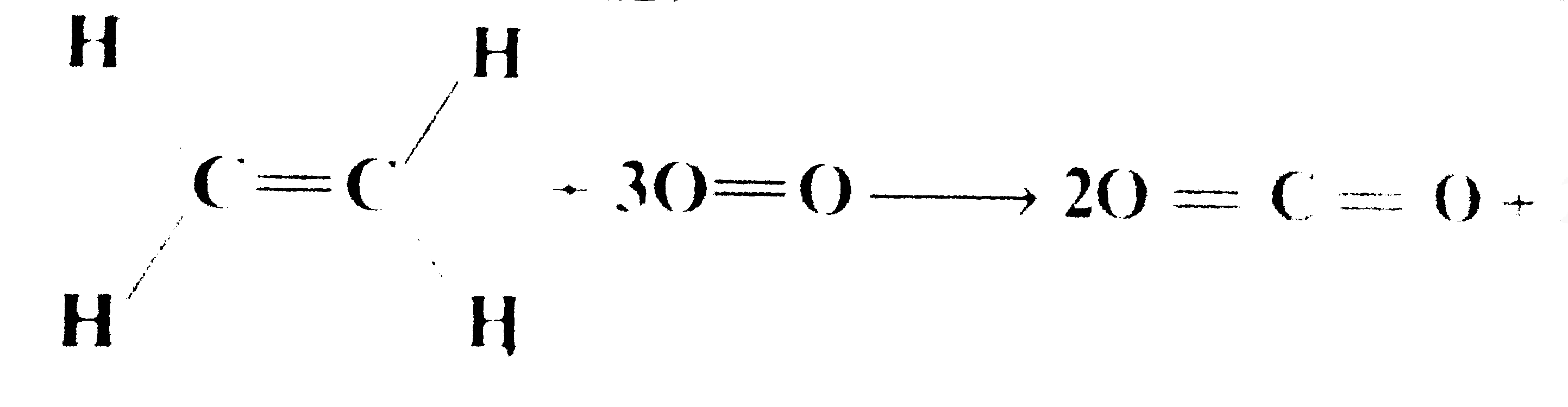Similar Questions
Explore conceptually related problems
Recommended Questions
- Calculate heat of combusion of ethene:
Text Solution
|
- Calculate the standard heat of formation of carbon disulphide (l). Giv...
Text Solution
|
- Calculate heat of combusion of ethene:
Text Solution
|
- The heat of combusion of benzene in a bomb calorimeter (i.e constant v...
Text Solution
|
- Merthyl bromide is converted into ethen by heating it in ether medium ...
Text Solution
|
- When 14 grams of ethene is completely burnt in oxygen, the heat evolve...
Text Solution
|
- The heat of combustion of ethylene at 18^(@)C and at constant volume i...
Text Solution
|
- The heat of combustion of benzene in a bomb calorimeter ( i.e., consta...
Text Solution
|
- In the combusion of 2g benzene, 2.5 kcal heat was evolved. What is the...
Text Solution
|