Similar Questions
Explore conceptually related problems
Recommended Questions
- An ideal monoatomic gas goes through a cyclic process. Ratio of maximu...
Text Solution
|
- One mole of an ideal gas is taken through a cyclic process. The minimu...
Text Solution
|
- An ideal gas goes through a cycle consisting of (a) isochoric, adiabat...
Text Solution
|
- An ideal gas goes through a cycle consisting of isothermal, polytropic...
Text Solution
|
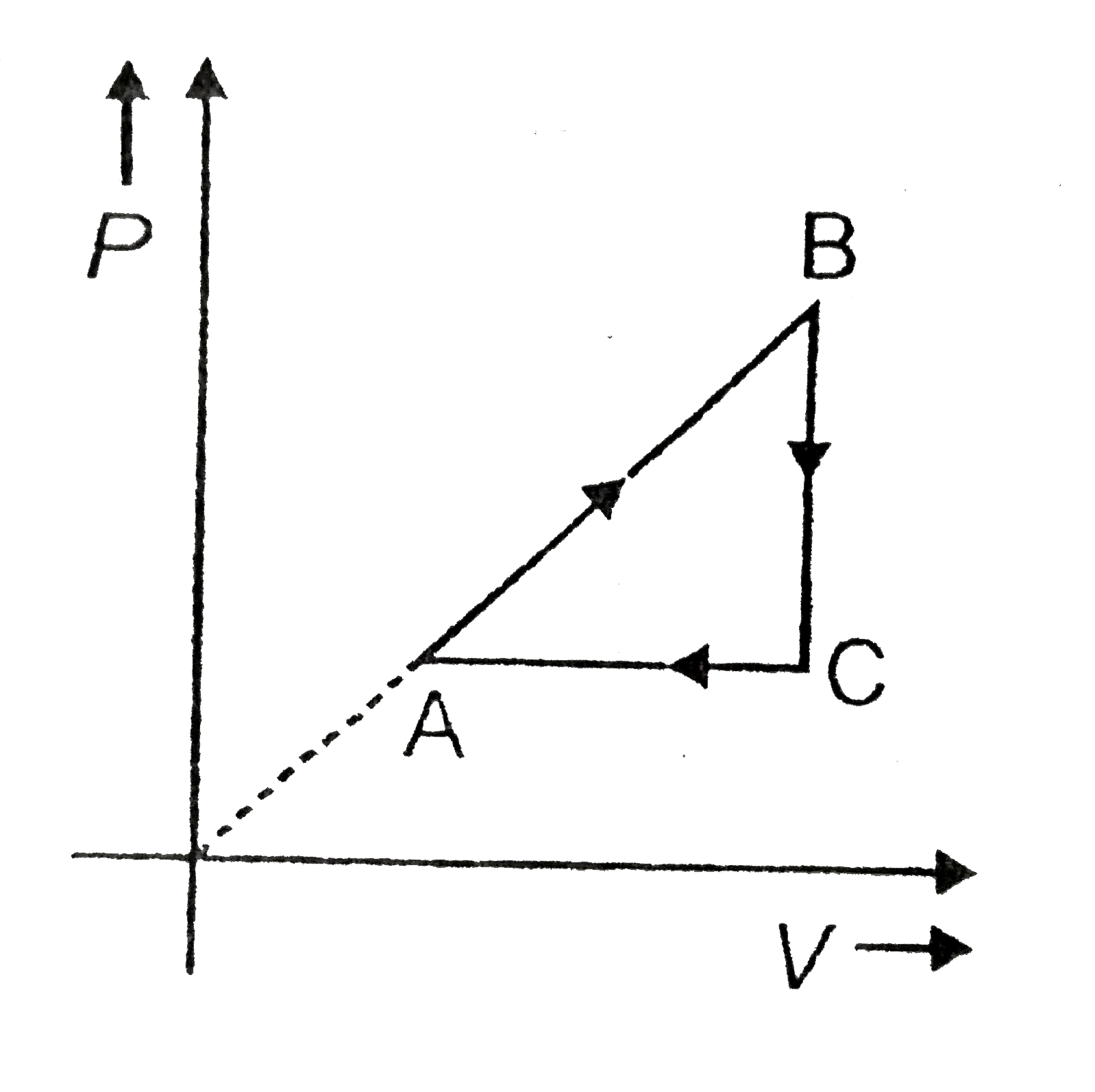
- An ideal gas with the adiabatic exponent gamma goes through a cycle (F...
Text Solution
|
- An ideal gas goes through a cycle consisting of ischoric adiabatic and...
Text Solution
|
- An ideal monoatomic gas (gamma=(5)/(3)) goes through a cyclic process ...
Text Solution
|
- An ideal gas is taken through cycle 1231 (see figure) and the efficien...
Text Solution
|
- An ideal gas undergoes a circular cycle as shown in the figure. Find t...
Text Solution
|
 .
.