Similar Questions
Explore conceptually related problems
Recommended Questions
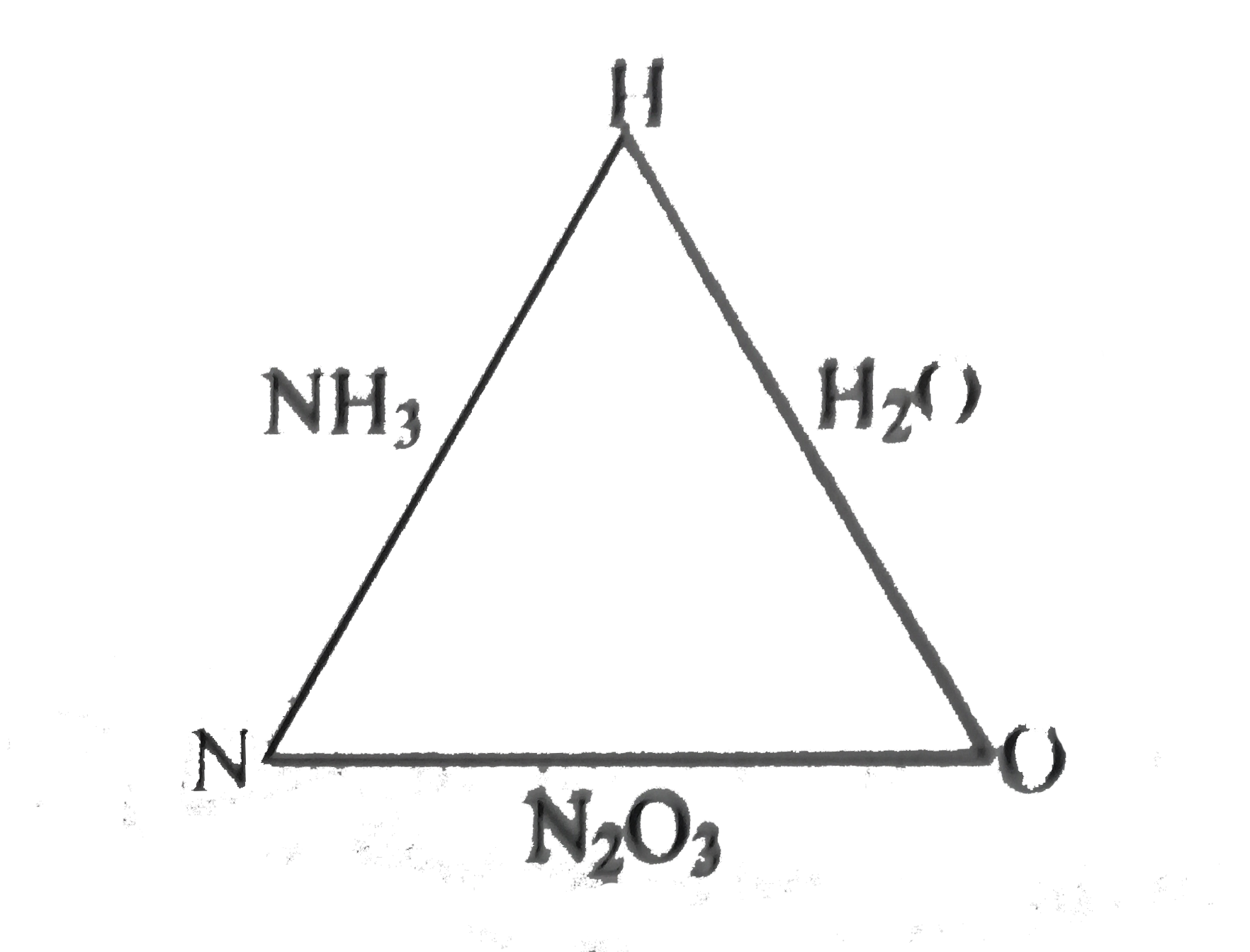
- Ammonia contains 82.35% of nitrogen and 17.65% of hydrogen. Water cont...
Text Solution
|
- Ammonia contains 82.35% of nitrogen and 17.65% of hydrogen. Water cont...
Text Solution
|
- Ammonia contains 82.32% of nitrogen and 17.65 % of hydrogen . Water co...
Text Solution
|
- are the organic compounds containing carbon, hydrogen, oxygen and nitr...
Text Solution
|
- NH(3) में 82.35% नाइट्रोजन तथा 17.65% हाइड्रोजन उपस्थित होती है. जल मे...
Text Solution
|
- Ammonia contains 82.35% of nitrogen and 17.65% of hydrogen. Water cont...
Text Solution
|
- Two oxides of nitrogen contain the following percentage compositions :...
Text Solution
|
- In compound A,1,.0g nitrogen combines with 0.57g oxygen. In compound B...
Text Solution
|
- Ammonia contains 17.65% of hydrogen, water contains 11.11% of hydrogen...
Text Solution
|