Similar Questions
Explore conceptually related problems
Recommended Questions
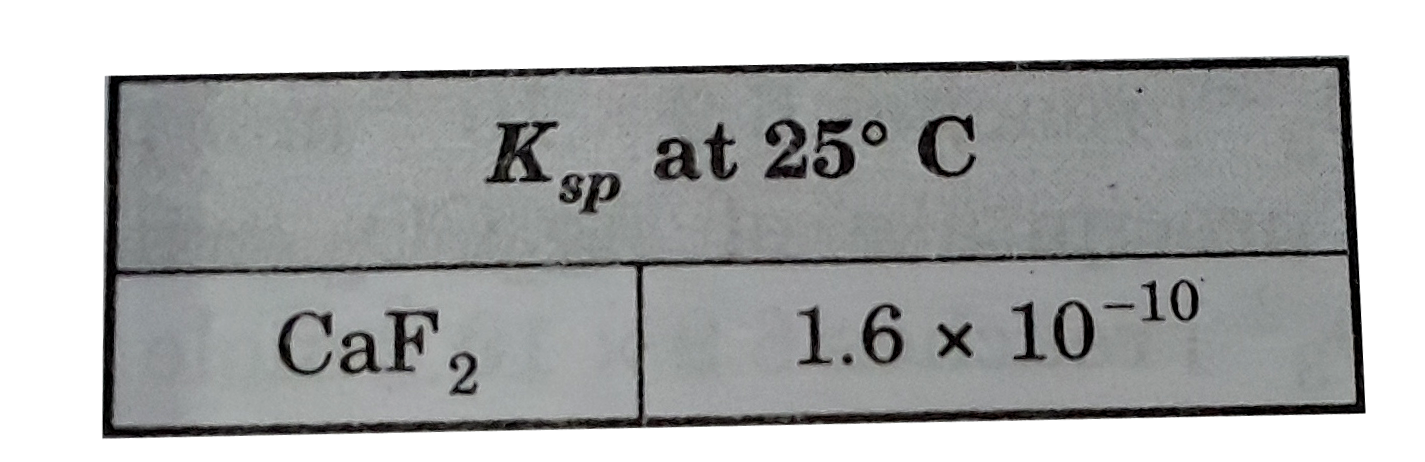
- How many moles of calcium fluoride , CaF(2), must be dissolved in 2.0 ...
Text Solution
|
- Calcium hydroxide is soluble in water with a K(sp) of 1.3xx10^(-6). Wh...
Text Solution
|
- CaF(2) has a K(sp)=3.9xx10^(-11) at 25^(@) C. What is the [F^(-)] in a...
Text Solution
|
- The K(sp) of calcium flouride is 3.2xx10^(-11). Calculate the DeltaG^(...
Text Solution
|
- How many moles of calcium fluoride , CaF(2), must be dissolved in 2.0 ...
Text Solution
|
- 4 g of a solute are dissolved in 40 g of water to form a saturated sol...
Text Solution
|
- The solubility of a saturated solution of calcium fluoride is 2xx10^(-...
Text Solution
|
- The co-ordination number of calcium fluoride (CaF(2)) type structure i...
Text Solution
|
- The number of formula units of calcium fluoride CaF(2) present in 146....
Text Solution
|