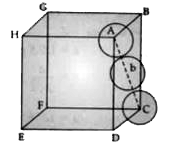
Text Solution
Verified by Experts
Topper's Solved these Questions
SOLID STATE
OSWAAL PUBLICATION|Exercise TOPIC -2 (VERY SHORT ANSWER TYPE QUESTIONS )|23 VideosSOLID STATE
OSWAAL PUBLICATION|Exercise TOPIC -2 (SHORT ANSWER TYPE QUESTIONS)|21 VideosSOLID STATE
OSWAAL PUBLICATION|Exercise TOPIC -1 (LONG ANSWER TYPE QUESTIONS - I)|12 VideosSample Paper 8
OSWAAL PUBLICATION|Exercise EXCERSICE|54 VideosSOLUTIONS
OSWAAL PUBLICATION|Exercise TOPIC - 3 COLLIGATIVE PROPERTIES , DETERMINATION OF MOLAR MASS, ABNORMAL MOLAR MASS, VAN.T HOFF FACTOR (LONG ANSWER TYPE QUESTIONS - II)|18 Videos
Similar Questions
Explore conceptually related problems