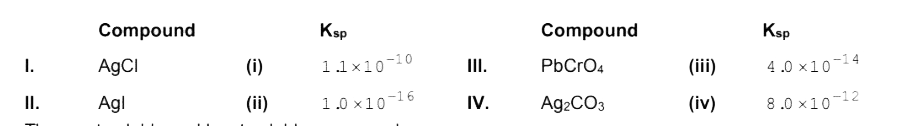Similar Questions
Explore conceptually related problems
Recommended Questions
- The solubility product (K(sp)) of the following compound are given at ...
Text Solution
|
- The solubility of a sparingly soluble compound MX(2) at 25^(@)C is 5.0...
Text Solution
|
- The solubility product (K(sp)) of the sparingly soluble salt MX at 25^...
Text Solution
|
- The solubility of product (K(sp)) of the following compounds are given...
Text Solution
|
- The solubility product (K(sp)) of the following compounds are given at...
Text Solution
|
- For which of the following sparingly soluble salt the solubility (s) a...
Text Solution
|
- The solubility product (K(sp)) of the following compounds are given at...
Text Solution
|
- 25^(@) C ताप पर यदि अलप विलेय लवण MX(2) का विलेयता गुणनफल K(sp) = 1.0 ...
Text Solution
|
- Incorrect order of solubility product (K(sp)) of given precipitated co...
Text Solution
|