Similar Questions
Explore conceptually related problems
Recommended Questions
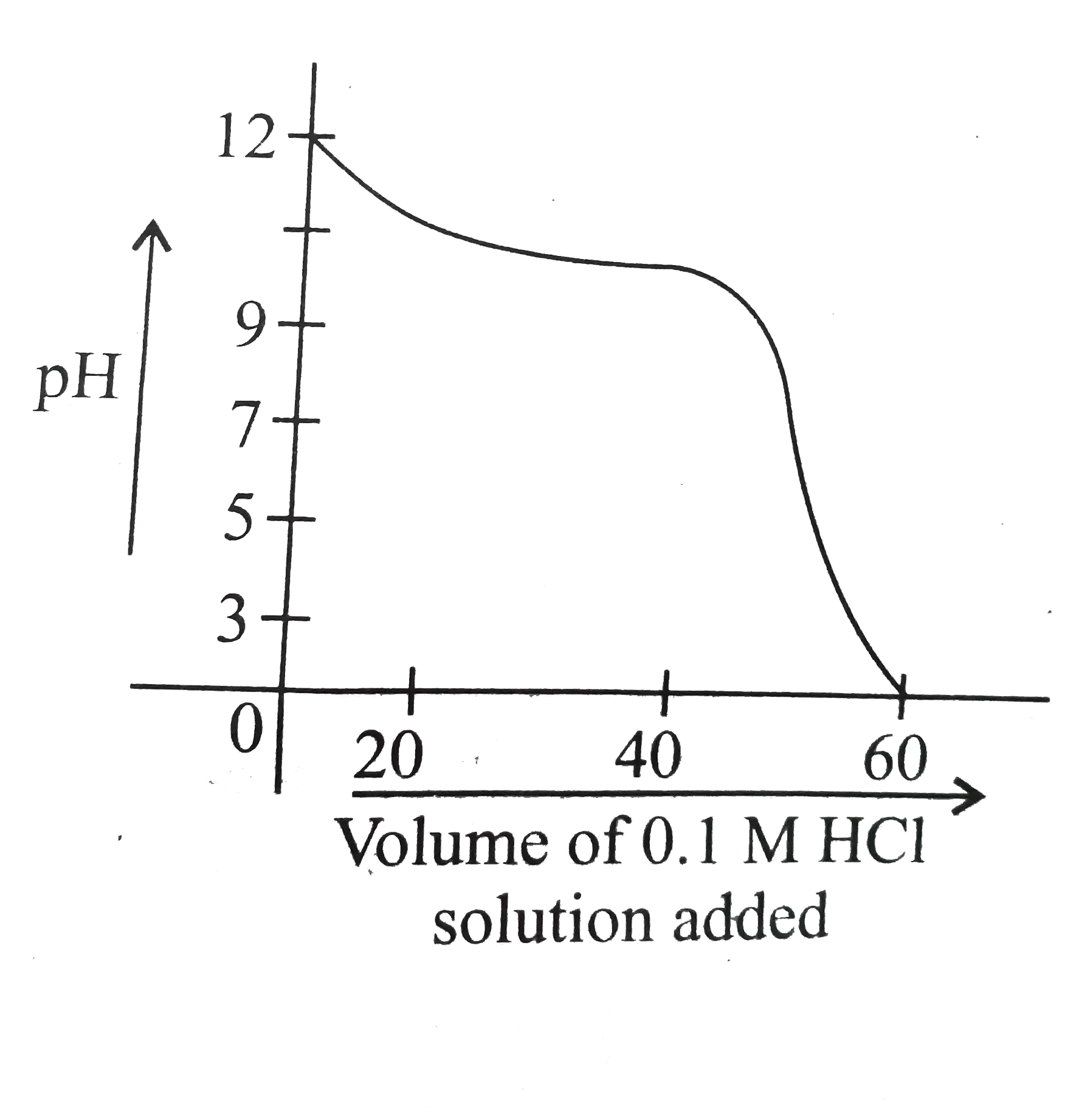
- When weak base solution (50mL of 0.1 N NH(4)OH) is titrated with stron...
Text Solution
|
- When weak base solution (50mL of 0.1 N NH(4)OH) is titrated with stron...
Text Solution
|
- When weak base solution (50 ml of 0.1 NH(4)OH ) is titrated with stron...
Text Solution
|
- A weak acid (or base) is titrated against a strong base (or acid), vol...
Text Solution
|
- A weak base (BOH) with K(b) = 10^(-5) is titrated with a strong acid (...
Text Solution
|
- In the titration of a weak acid of known concentratin with a standard ...
Text Solution
|
- In the titration of weak acid against strong base match the pH of the ...
Text Solution
|
- A solution of a substances is titrated against a strong base ( or acid...
Text Solution
|
- When a strong acid is titrated using a weak base, the pH at the equiva...
Text Solution
|