Similar Questions
Explore conceptually related problems
Recommended Questions
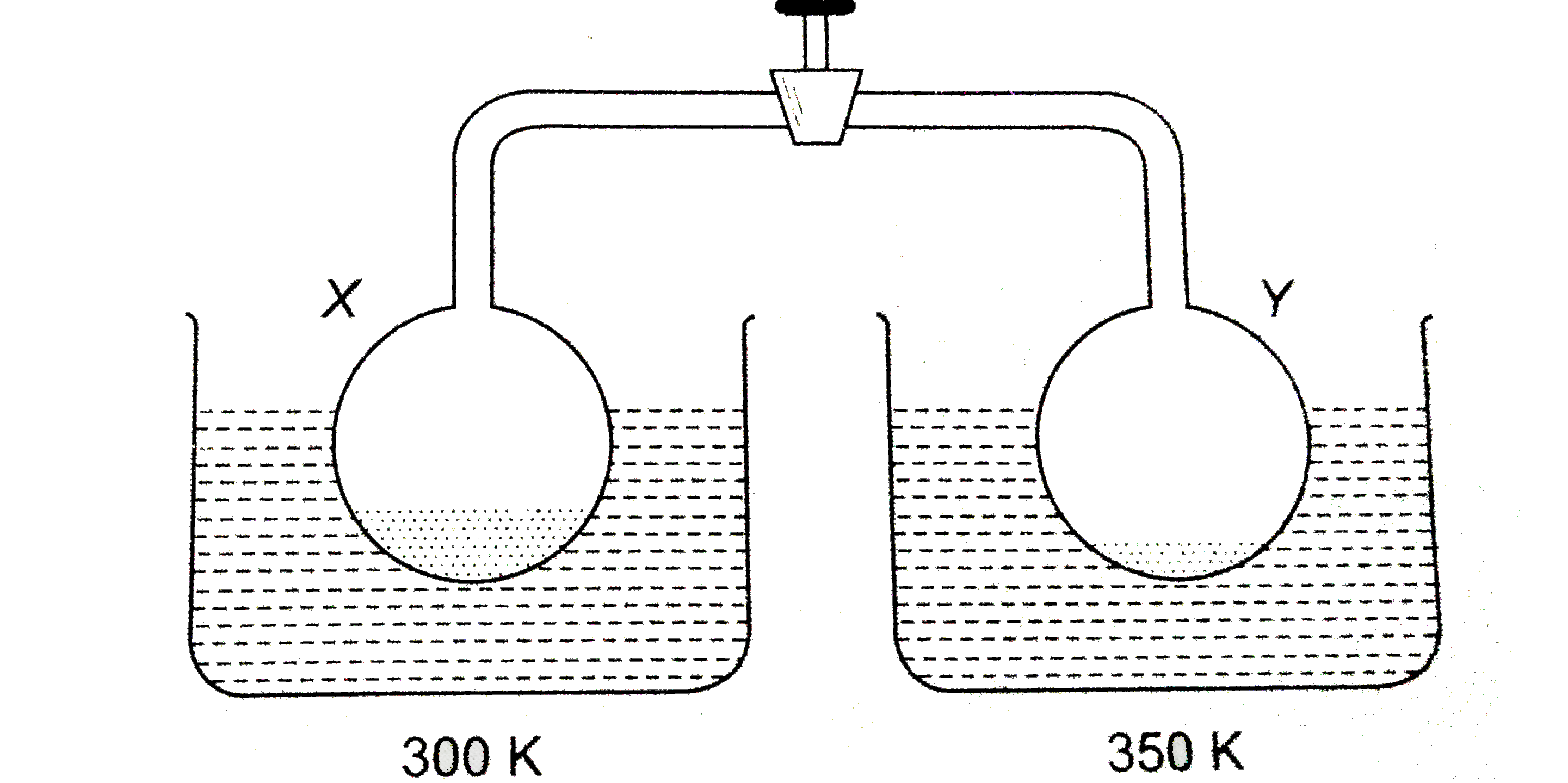
- Two container each containing liquid water are connected as shown in d...
Text Solution
|
- A sample of air contains only N(2), O(2), and H(2)O. It saturated with...
Text Solution
|
- Two container each containing liquid water are connected as shown in d...
Text Solution
|
- A sample of air contains only N(2)O(2) and H(2)O. It is saturated with...
Text Solution
|
- A container contains air above liquid water. Total pressure was 800 to...
Text Solution
|
- Given at 350 K, P(A)^(@) = 300 torr and P(B)^(@) =800 torr the composi...
Text Solution
|
- 821mLN(2) (g) was collected over liquid water at 300 K and 1 atm.If va...
Text Solution
|
- Two container each containing liquid water are connected as shown in d...
Text Solution
|
- The molar heat of vapourization of toluene is DeltaHv if its vapour pr...
Text Solution
|