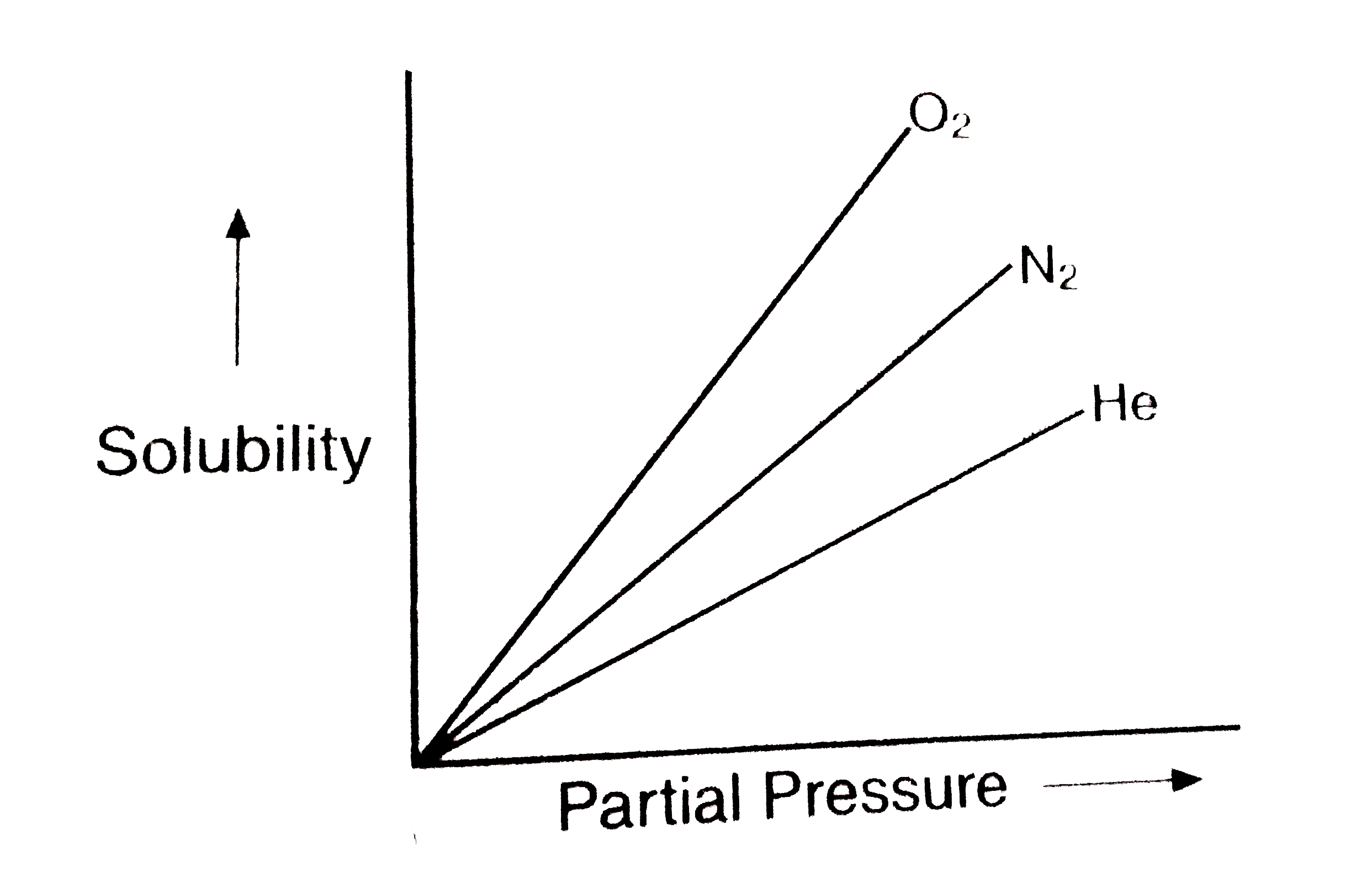Similar Questions
Explore conceptually related problems
Recommended Questions
- Molar solubility of helium, nitrogen and oxygen are plotted against pa...
Text Solution
|
- Graph of ln S^(@) vs (1)/(T) is plotted for two gases A and B [S^(@) r...
Text Solution
|
- Molar solubility of helium, nitrogen and oxygen are plotted against pa...
Text Solution
|
- Solubility of oxygen gas in water follows Henry's law. When the solubi...
Text Solution
|
- According to Henry's law the partial pressure of the gas in vapour pha...
Text Solution
|
- Which one of the following gases has the lowest value of Henry's law c...
Text Solution
|
- Which one of the following gases has the lowest value of Henry law con...
Text Solution
|
- How is the solubility of gases in water related with their Henry's con...
Text Solution
|
- Assertion. Greater the value of Henry's constant of a gas in a particu...
Text Solution
|