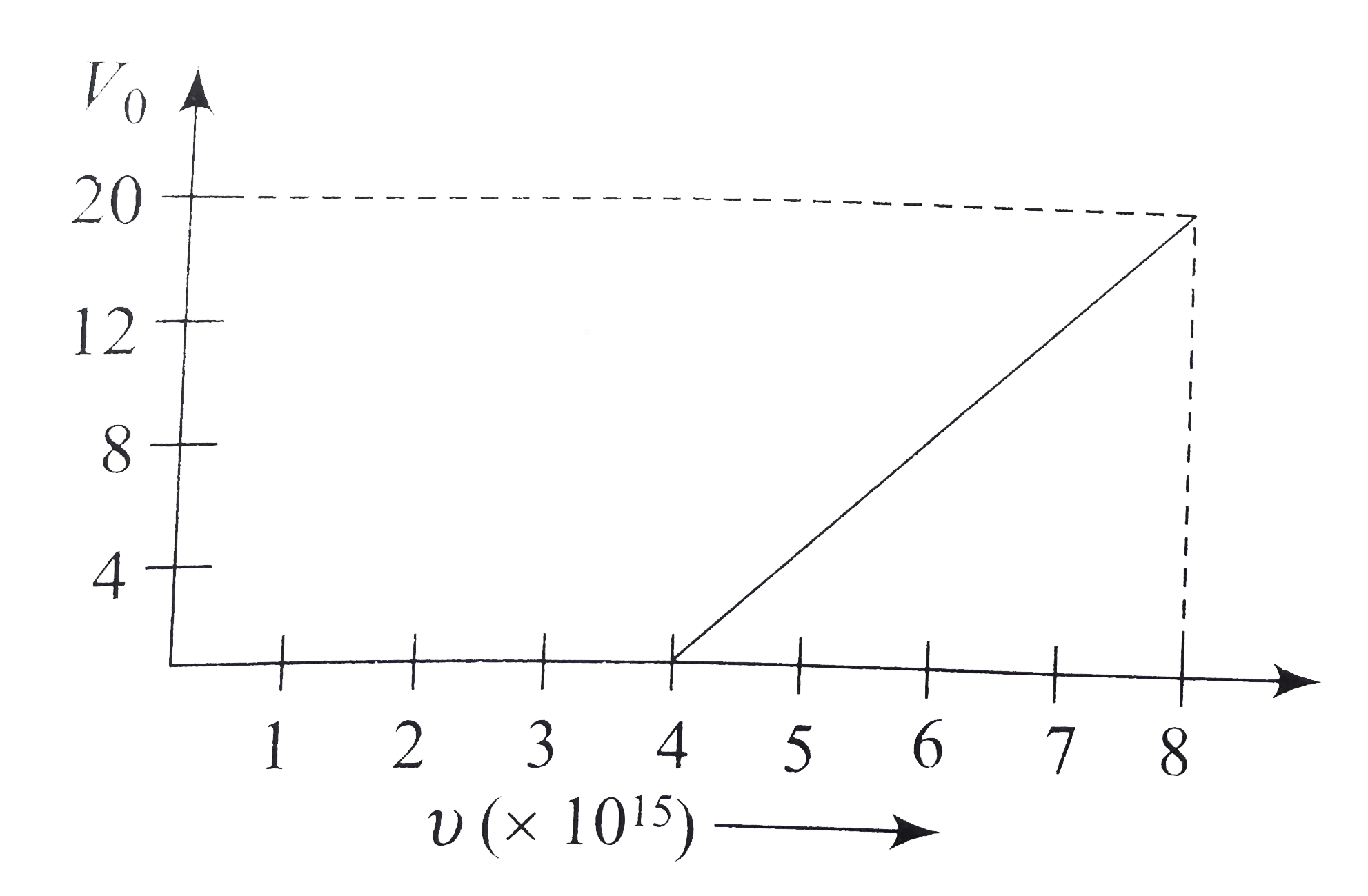Similar Questions
Explore conceptually related problems
Recommended Questions
- When a high frequency electromagnetic radiation is incident on a metal...
Text Solution
|
- When light of sufficiently high frequency is incident on a metallic su...
Text Solution
|
- When light of sufficiently high frequency is incident on a metallic su...
Text Solution
|
- When a high frequency electromagnetic radiation is incident on a metal...
Text Solution
|
- When a high frequency electromagnetic radiation is incident on a metal...
Text Solution
|
- When a high frequency electromagnetic radiation is incident on a metal...
Text Solution
|
- Assertion (A) : On increasing the frequency of light larger number of ...
Text Solution
|
- प्रकाश-विद्युत प्रभाव में किसी धातु के पृष्ठ से प्रति सेकण्ड उत्सर्जित...
Text Solution
|
- जब प्रकाश किसी धातु की सतह पर आपतित होता है, तो एक फोटॉन केवल एक इलेक्...
Text Solution
|