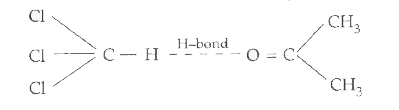Text Solution
Verified by Experts
Topper's Solved these Questions
SOLUTIONS
OSWAAL PUBLICATION|Exercise TOPIC - 3 COLLIGATIVE PROPERTIES , DETERMINATION OF MOLAR MASS, ABNORMAL MOLAR MASS, VAN.T HOFF FACTOR (LONG ANSWER TYPE QUESTIONS - I)|17 VideosSOLID STATE
OSWAAL PUBLICATION|Exercise TOPIC -2 (SHORT ANSWER TYPE QUESTIONS)|21 VideosSolved Paper
OSWAAL PUBLICATION|Exercise Exercise|60 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-SOLUTIONS-TOPIC - 3 COLLIGATIVE PROPERTIES , DETERMINATION OF MOLAR MASS, ABNORMAL MOLAR MASS, VAN.T HOFF FACTOR (LONG ANSWER TYPE QUESTIONS - II)
- (a) Define the following terms : (i) Molarity (ii) Molal elevati...
Text Solution
|
- (a) What type of deviation is shown by a mixture of ethanol and aceton...
Text Solution
|
- (a) The vapour pressures of benzene and toluene at 293 Kare 75 mm Hg a...
Text Solution
|
- (a) A 5% solution (by mass) of cane - sugar in water has freezing poin...
Text Solution
|
- (a) Define the following terms : (i) Ideal solution (ii) Azeotrope...
Text Solution
|
- 15 g of an unknown molecular substance was dissolved in 450 g of water...
Text Solution
|
- Define the terms osmosis and osmotic pressutt. Is the osmotic pressure...
Text Solution
|
- Calculate the boiling point of a solution prepared by adding 15.00 g o...
Text Solution
|
- Differentiate between molarity and molality in a solution. What is the...
Text Solution
|
- What would be the molar mass of a compound if I 6.21 g of it dissolved...
Text Solution
|
- What is Van't Hoff factor ? What possible values can it have If the so...
Text Solution
|
- An aqueous solution containing 12.48 g of barium chloride in 1.0 kg of...
Text Solution
|
- List any four factors on which the colligative properties of a solutio...
Text Solution
|
- Calculate the bolling point of one mole aqueous solution (density 1.06...
Text Solution
|
- Explain why a solution of chloroform and acetone shows negative deviat...
Text Solution
|
- Phenol associates in benzene to a certain extent to form a dimer. A so...
Text Solution
|
- What is Van't Hoff factor ? Under what conditions Van't Hoff factor is...
Text Solution
|
- What will happen to the boiling point of a solution if mass of the sol...
Text Solution
|