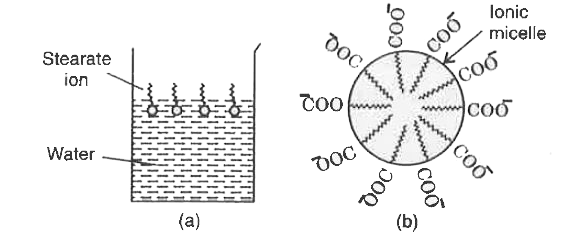
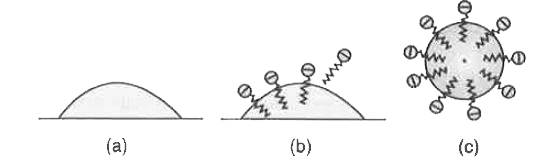
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-SURFACE CHEMISTRY -TOPIC - 3 (COLLOIDS, TYPES OF COLLOIDS, CHARACTERISTICS AND PREPARATION OF COLLOIDS)(LONG ANSWER TYPE QUESTIONS-II)
- (a) Complete and balance the following reaction (i) SO(2) + H2S over...
Text Solution
|
- (a) What is coagulation of sol ? name two methods by which a lyophobic...
Text Solution
|
- (a) What is (a) multimolecular colloid (b) macromolecular colloid and ...
Text Solution
|
- (a) Describe electrophoresis with the help of a diagram. (b) What is...
Text Solution
|
- (a) Give any three differences between physical adsorption and chemica...
Text Solution
|
- (a) Give any three differences between Physisorption and chemisorption...
Text Solution
|
- What are lyophilic and lyophobic sols ? Give are example of each type....
Text Solution
|
- What is the difference between multimolecular and macromolecular collo...
Text Solution
|
- Action of soap is due to emulsification and micelle formation, Comment...
Text Solution
|
- Explain the following terms: (i) Electrophoresis (ii) Coagulation (i...
Text Solution
|
- A house wife while working in the kitchen get a cut on the finger. It ...
Text Solution
|
- Radha and Meera are fast friends. They both study in class XII. Radha ...
Text Solution
|
- Reetu is purely vegetarian but her husband Rohit likes non-vegetarian ...
Text Solution
|
- On visit to your native village, you find that lot of smoke, dust and ...
Text Solution
|