Similar Questions
Explore conceptually related problems
Recommended Questions
- Calculate the Enthalpy of hydrogenation of If the Delta(f)H of and a...
Text Solution
|
- Calculate the enthalpy of combustion of benzene (l) on the basis of th...
Text Solution
|
- Delta(f)H^(Theta) of Cyclohexene (l) and benzene at 25^(@)C is -156 an...
Text Solution
|
- Given the bond energies of H - H and Cl - Cl are 430 kJ mol^(-1) and 2...
Text Solution
|
- Calculate the Enthalpy of hydrogenation of If the Delta(f) H of and ar...
Text Solution
|
- Use the following data to calculate Delta("lattice") H^(Θ) " for " NaB...
Text Solution
|
- Given the bond energies of H - H and Cl - Cl are 430 kJ mol^(-1) and 2...
Text Solution
|
- Calculate the bond enthalpy of HCl. Given that the bond enthalpies of ...
Text Solution
|
- Calculate the enthalpy change for the process C Cl(4)(g) rarr C(g)+4...
Text Solution
|
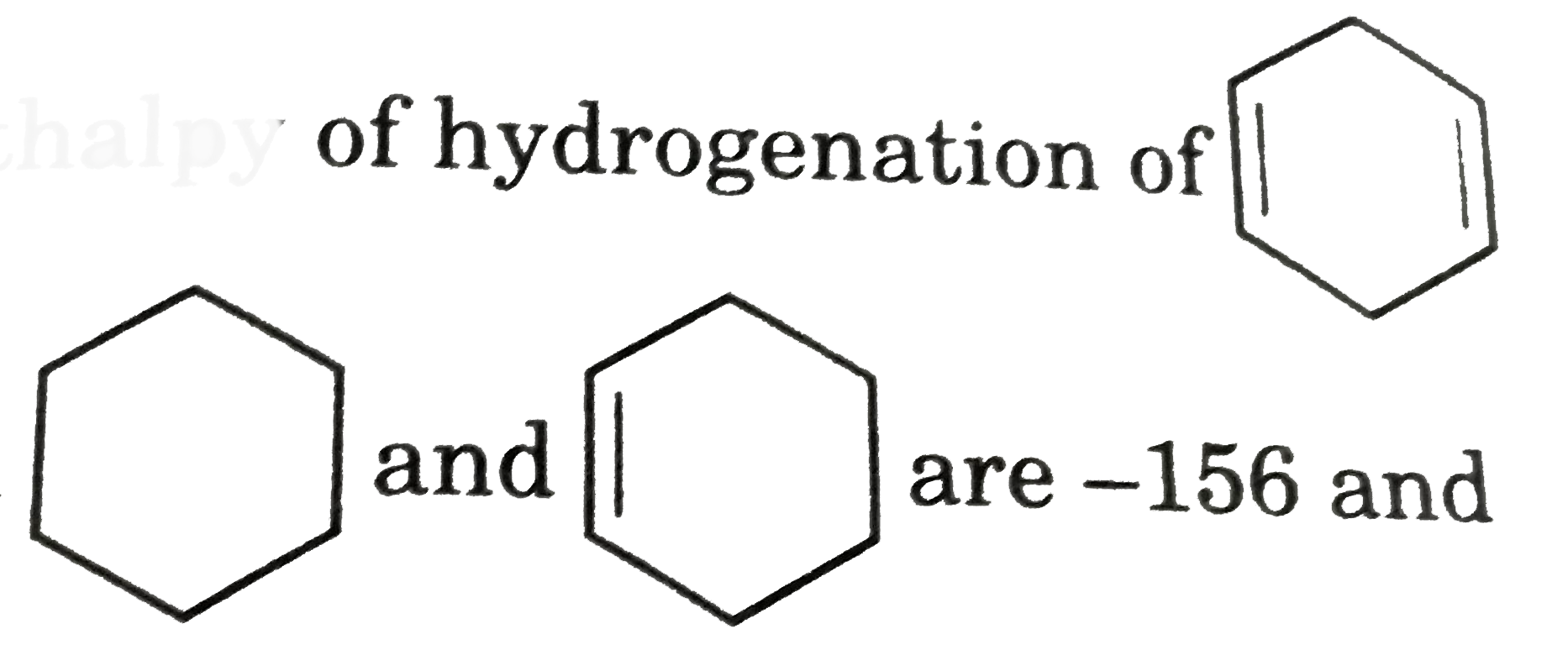 are -156 and -37 Kj /mol respectively.
are -156 and -37 Kj /mol respectively.