Similar Questions
Explore conceptually related problems
Recommended Questions
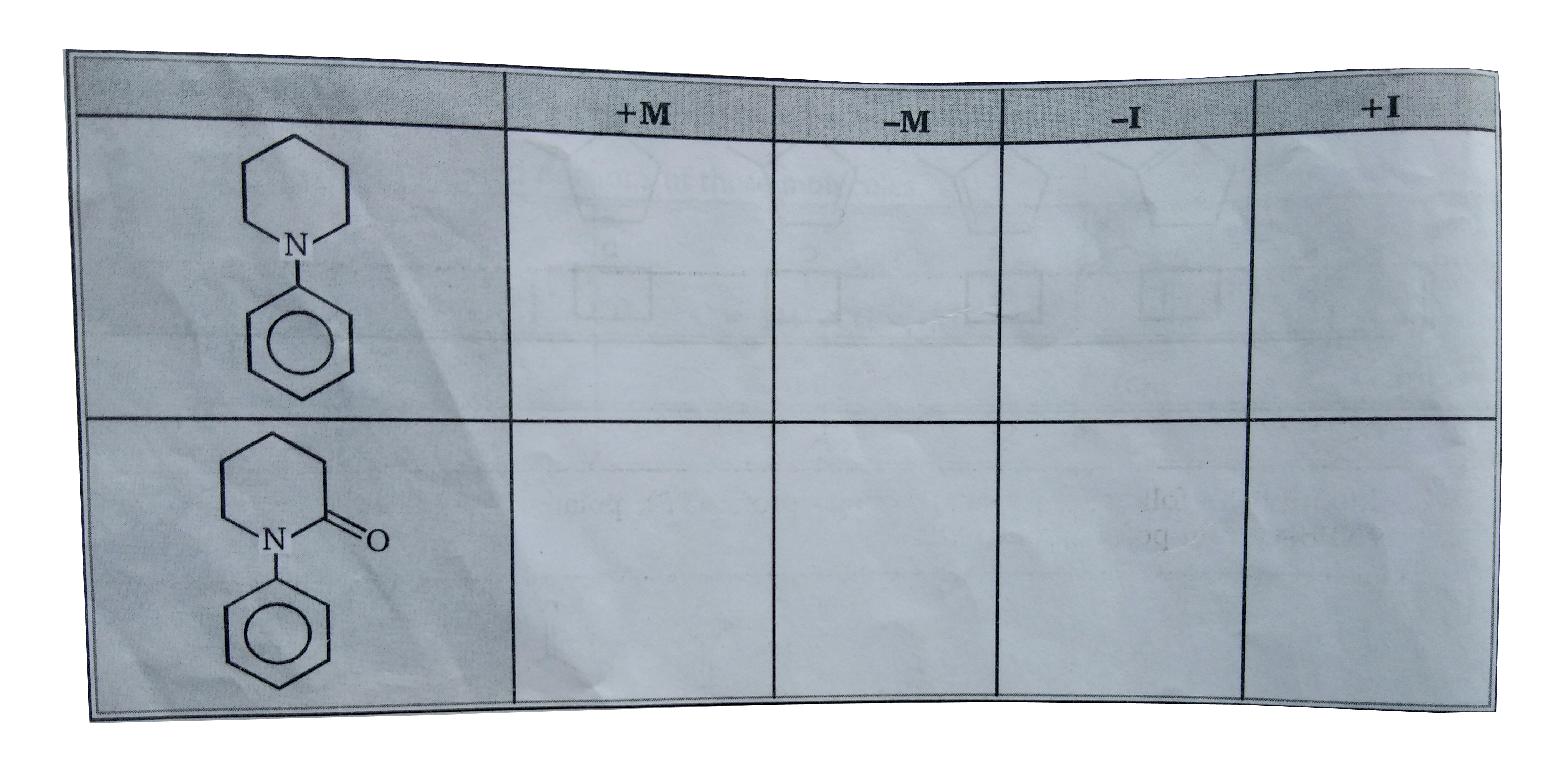
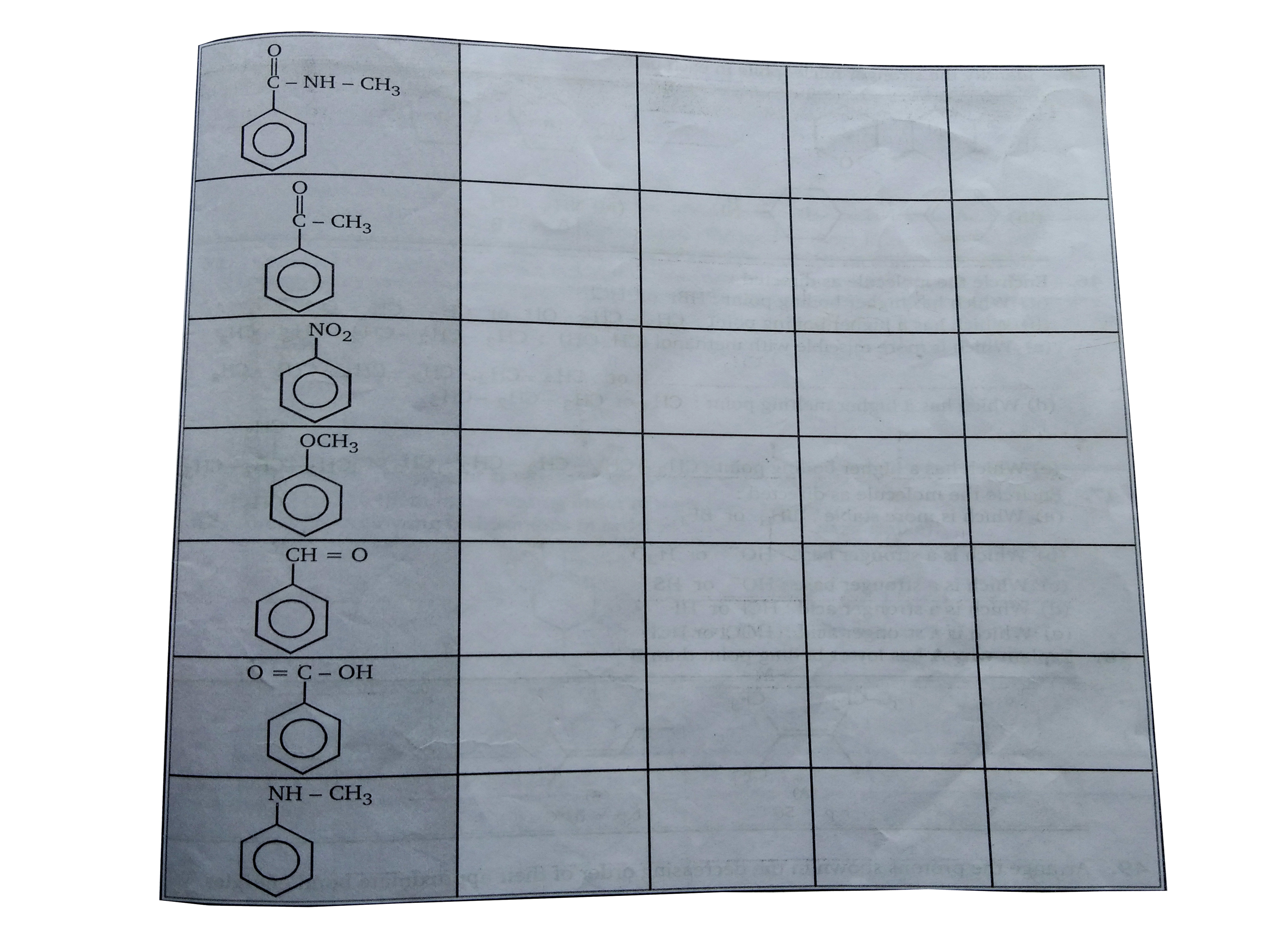
- Indetify (+M) mesomeric &(-M) group of following
Text Solution
|
- MESOMERIC EFFECT (+M)
Text Solution
|
- MESOMERIC EFFECT (-M)
Text Solution
|
- Which of the following have +M effect ( overline e - donating mesomeri...
Text Solution
|
- Which of the following have -M effect ( overline e - withdrawing mesom...
Text Solution
|
- Indetify the group of plants possessing leaf tendrils
Text Solution
|
- Indetify (+M) mesomeric &(-M) group of following
Text Solution
|
- Which of the following series contains atoms/groups having only -M(me...
Text Solution
|
- मेसोमेरिक प्रभाव को परिभाषित कीजिए तथा +M एवं -M प्रभाव के मध्य विभेद ...
Text Solution
|