Similar Questions
Explore conceptually related problems
Recommended Questions
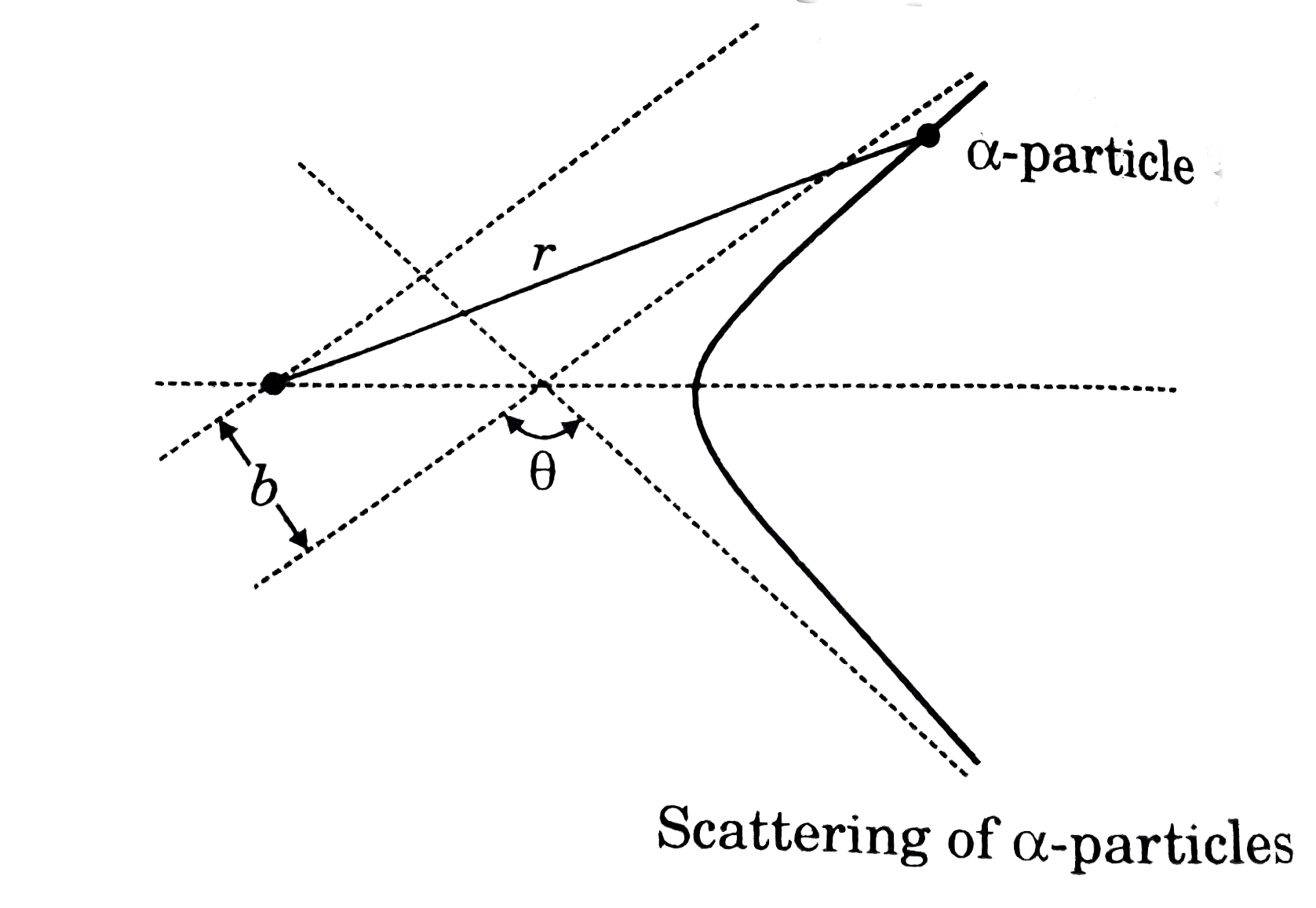
- The following text gives a qualitative idea about Rutherford's alpha- ...
Text Solution
|
- for scattering by an inverse square law field (such as that produced b...
Text Solution
|
- The following text gives a qualitative idea about Rutherford's alpha- ...
Text Solution
|
- The following text gives a qualitative idea about Rutherford's alpha- ...
Text Solution
|
- What is the impact parameter at which the scattering angle is 10^(@) f...
Text Solution
|
- In Rutherford scattering experiment, what will be the correct angle fo...
Text Solution
|
- In Rutherford experiment alpha – particles are scattered by nucleus ha...
Text Solution
|
- In Rutherford experiment, for head-on collision of alpha particles wi...
Text Solution
|
- In Rutherford alpha -sattering experiment , the ratio of number of par...
Text Solution
|