Similar Questions
Explore conceptually related problems
Recommended Questions
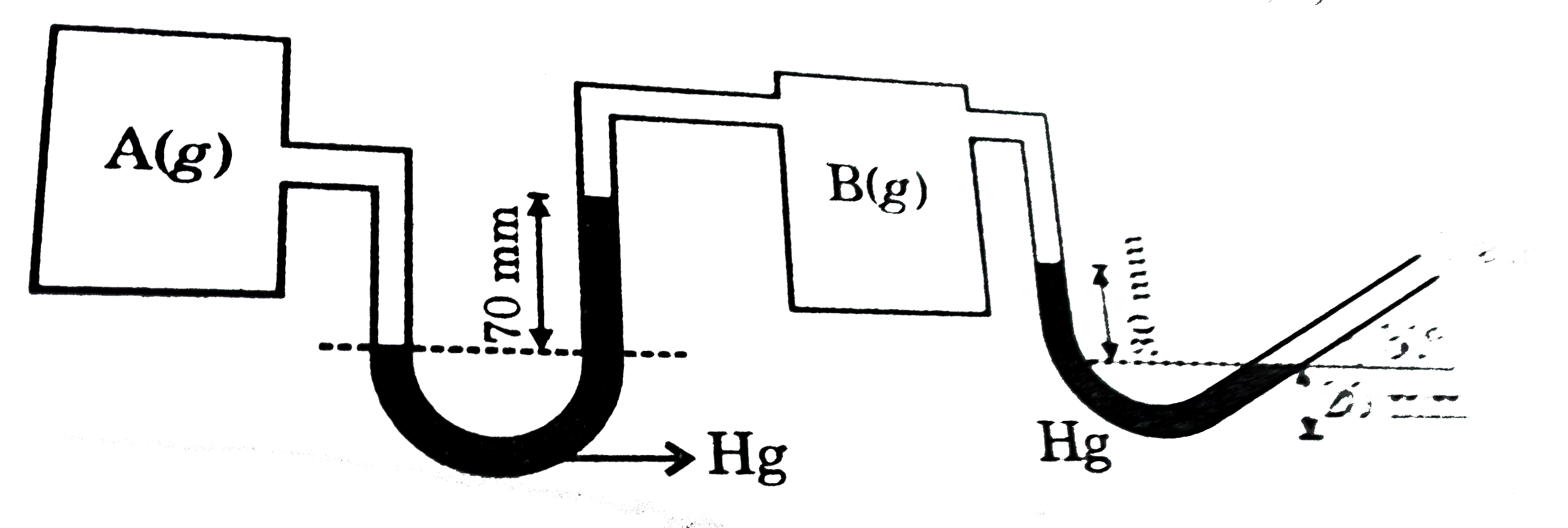
- At 300 K two gases are filled in two equal sized containers as given ...
Text Solution
|
- At 300 K , two gases are filled in two equal sized containers as given...
Text Solution
|
- At 300 K two gases are filled in two equal sized containers as given ...
Text Solution
|
- At 300 K the vapour pressure of two pure liquids, A and B are 100 and ...
Text Solution
|
- A closed container contains equal number of moles of two gases X and Y...
Text Solution
|
- At a constant volume, a quantity of an ideal gas has a pressure of 80...
Text Solution
|
- Two liquids A and B form an ideal solution. At 300 K, the vapour press...
Text Solution
|
- At 300 K two pure liquids A and B have vapour pressures respectively 1...
Text Solution
|
- At 300 K two pure liquids A and B have vapour pressures respectively 1...
Text Solution
|