Similar Questions
Explore conceptually related problems
Recommended Questions
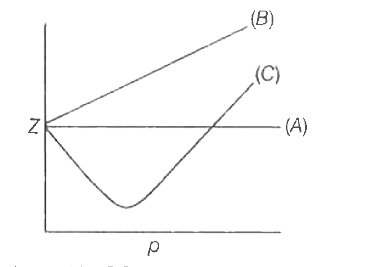
- The variation of compressibility factor (Z) with pressure (p in bar) ...
Text Solution
|
- The given graph represents the variations of compressibility factor Z=...
Text Solution
|
- The graphs in Fig shown the variation of the product PV with respect t...
Text Solution
|
- Figure displays the plot of the compression factor Z versus P for a fe...
Text Solution
|
- Consider the graph between compressibility factor Z and pressure P, Th...
Text Solution
|
- The variation of compressibility factor (Z) with pressure (p in bar) ...
Text Solution
|
- The given graph represents the variations of compressibility factor Z=...
Text Solution
|
- Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure ...
Text Solution
|
- The given graph represents the variation of Z (compressibility factor ...
Text Solution
|