Similar Questions
Explore conceptually related problems
Recommended Questions
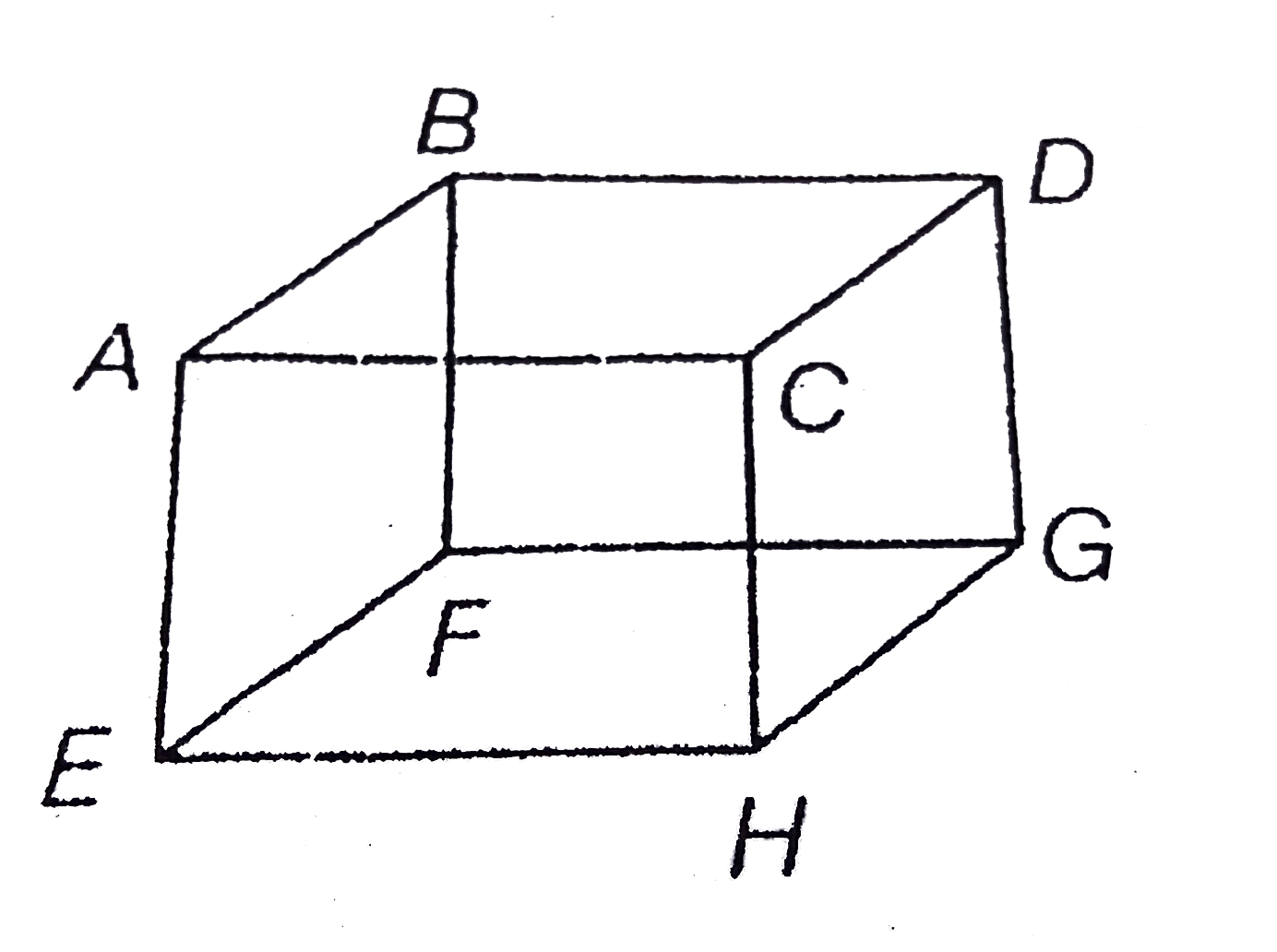
- Mole of an ideal gas is contained in a cubical volume V, ABCDEFGH at 3...
Text Solution
|
- Mole of an ideal gas is contained in a cubical volume V, ABCDEFGH at 3...
Text Solution
|
- Mole of an ideal gas is contained in a cubical volume V, ABCDEFGH at 3...
Text Solution
|
- आदर्श गैस से आप क्या समझते है ? गैस के किसी एक मोल के लिये आदर्श गैस स...
Text Solution
|
- Container A holds an ideal gas at a pressure 1 xx10^5 Pa and at 300 K...
Text Solution
|
- Container A holds an ideal gas at a pressure 1 xx10^5 Pa and at 300 K....
Text Solution
|
- Kinetic energy of one mole of an ideal gas at 300 K in kJ is
Text Solution
|
- One mole of an ideal gas at 300 K expands from V to 2V volume, then wo...
Text Solution
|
- The volume of one mole of an ideal gas changes from V to 2V at tempera...
Text Solution
|