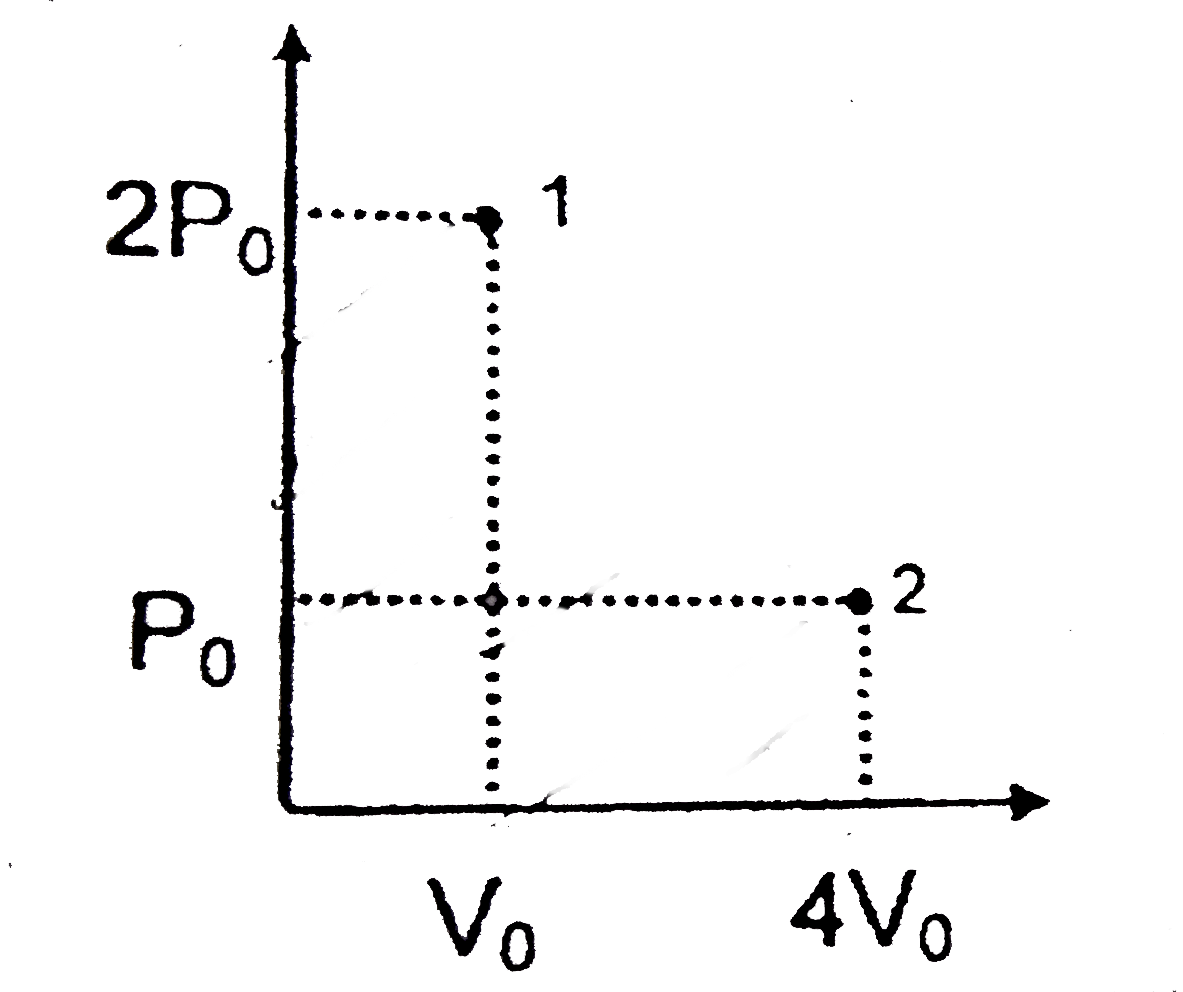Similar Questions
Explore conceptually related problems
Recommended Questions
- A liquid confined inside an adiabatic is suddenly taken from state 1 t...
Text Solution
|
- A liquid confined inside an adiabatic is suddenly taken from state 1 t...
Text Solution
|
- An ideal mono atomic gas is taken through the cyclic process shown in ...
Text Solution
|
- A liquid which is confined inside an adiabatic piston is suddently tak...
Text Solution
|
- An ideal gas taken from state A(P,V) to the state B(P//2,2V) along a s...
Text Solution
|
- One mole of an ideal monoatomic gas undergo process from the state ...
Text Solution
|
- A liquid which is confined inside an adiabatic piston is suddenly take...
Text Solution
|
- For an ideal gas three adiabatic processes are carried out upto same f...
Text Solution
|
- An ideal gas is taken from the same initial pressure P(1) to the same ...
Text Solution
|