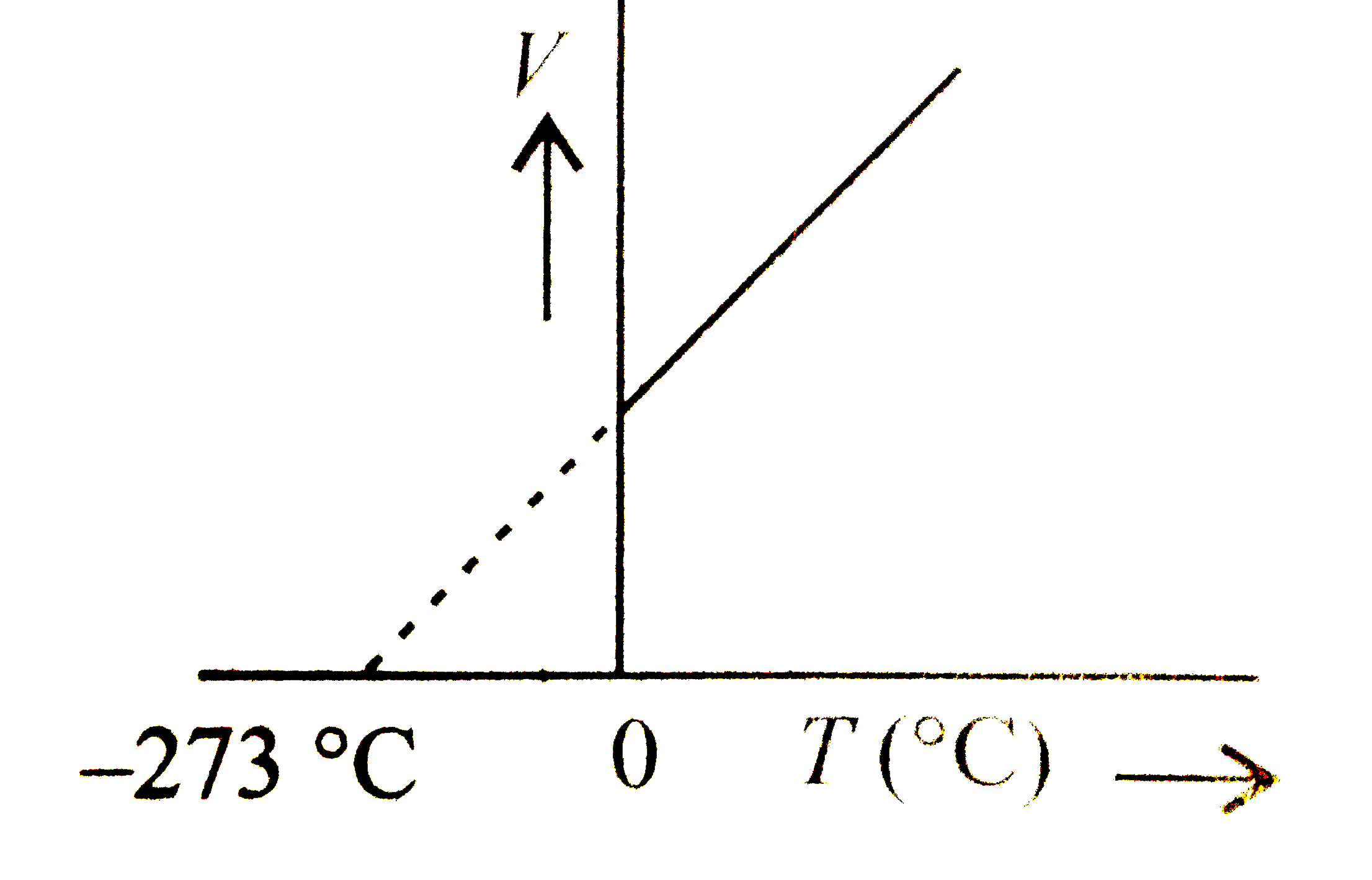Similar Questions
Explore conceptually related problems
Recommended Questions
- If we plot volume of a certain mass of a gas against temperature at co...
Text Solution
|
- The temperature of a certain mass of a gas increased from 37^(@)C to 3...
Text Solution
|
- Pressure versus temperature graph of an ideal gas at constant volume V...
Text Solution
|
- कथन - स्थिर दाब पर किसी गैस के निश्चित द्रव्यमान का आयतन इसके परमताप ...
Text Solution
|
- If we plot volume of a certain mass of a gas against temperature at co...
Text Solution
|
- স্থির চাপে কোন নির্দিষ্ট ভরের গ্যাসের আয়তন উষ্ণতার সঙ্গে
Text Solution
|
- नियत दाब पर, किसी गैस के निश्चित द्रव्यमान का आयतन उसके परम ताप का होत...
Text Solution
|
- If we draw a graph of the volume of a gas of a certain mass against te...
Text Solution
|
- Explain constant volume gas thermometer and absolute zero temperature.
Text Solution
|