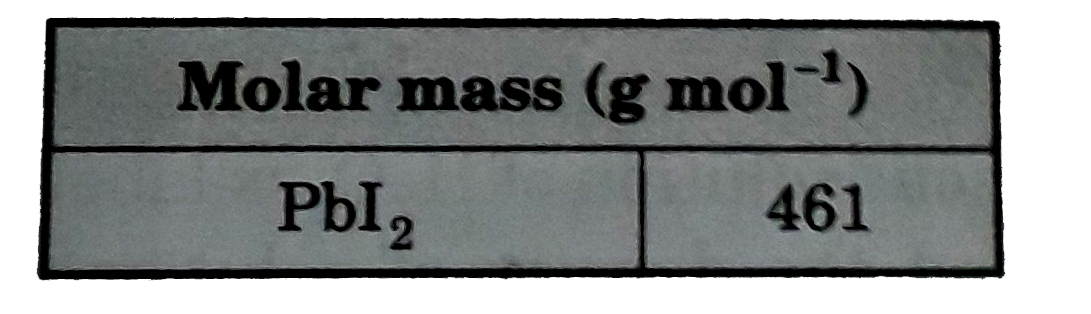Similar Questions
Explore conceptually related problems
Recommended Questions
- What is the maximum mass of PbI(2) that can be precipitated by mixing ...
Text Solution
|
- 25.0 mL clear saturated solution of PbI(2(aq.)) requires 13.3mL of AgN...
Text Solution
|
- If 100 mL of 0.100 M HCl solution and 100 mL of 0.100 M NaOH are mixed...
Text Solution
|
- Pb(NO(3))(2) and KI reacts in aqueous solution to form an yellow preci...
Text Solution
|
- 400 ml of 0.2 M-HCl is mixed with 600 ml of 0.1 M-NaOH solution. The m...
Text Solution
|
- What is the concentration of the solution that results from mixing 40....
Text Solution
|
- A 50.0 mL solution of 0.150 M HCl. Is mixed with 25.0 mL of 0.400 M HC...
Text Solution
|
- What is the maximum mass of PbI(2) that can be precipitated by mixing ...
Text Solution
|
- A solution is prepared by mixing 25.0 mL of 6.0 M HCI with 45.0 mL of ...
Text Solution
|