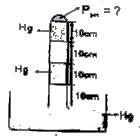Similar Questions
Explore conceptually related problems
Recommended Questions
- In the above figure mercury columns of 10 cms each are trapped between...
Text Solution
|
- In the above figure mercury columns of 10 cms each are trapped between...
Text Solution
|
- A glass tube with a sealed end is completely submerged in a vessel wit...
Text Solution
|
- An ideal gas is trapped between a mercury column and the closed lower ...
Text Solution
|
- A tube of length 50 cm is containing a gas in two secitons separated b...
Text Solution
|
- स्थिर आयतन पर बल्ब में भरी गैस का 0^@C पर दाब 66 सेमी (पारा स्तम्भ) तथ...
Text Solution
|
- A gas at certain volume and temperature has a pressure equal to 75 cm ...
Text Solution
|
- 76 सेमी पारे के स्तम्भ के दाब के बराबर जल के स्तम्भ की ऊँचाई ज्ञात कीज...
Text Solution
|
- In the given figure, atmospheric pressure p0 = 1 atm and mercury colum...
Text Solution
|