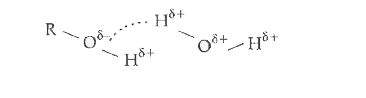
Text Solution
Verified by Experts
Topper's Solved these Questions
ALCOHOLS, PHENOLS AND ETHERS
OSWAAL PUBLICATION|Exercise TOPIC -1 ALCOHOLS, PHENOLS AND ETHERS (LONG ANSWER TYPE QUESTIONS -II)|1 VideosALCOHOLS, PHENOLS AND ETHERS
OSWAAL PUBLICATION|Exercise TOPIC -2 METHODS OF PREPARATION OF ALKANOLS (VERY SHORT ANSWER TYPE QUESTIONS)|5 VideosALCOHOLS, PHENOLS AND ETHERS
OSWAAL PUBLICATION|Exercise TOPIC -1 ALCOHOLS, PHENOLS AND ETHERS ( SHORT ANSWER TYPE QUESTIONS)|2 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
OSWAAL PUBLICATION|Exercise TOPIC -3 METHODS OF PREPARATION OF CARBOXYLIC ACID, PROPERTIES AND USES (LONG ANSWER TYPE QUESTIONS II)|7 Videos
Similar Questions
Explore conceptually related problems