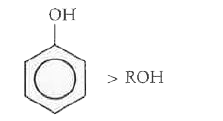
Text Solution
Verified by Experts
Topper's Solved these Questions
ALCOHOLS, PHENOLS AND ETHERS
OSWAAL PUBLICATION|Exercise TOPIC -4 CLASSIFICATION AND NOMENCLATURE OF ETHERS (LONG ANSWER TYPE QUESTION -I)|1 VideosALCOHOLS, PHENOLS AND ETHERS
OSWAAL PUBLICATION|Exercise TOPIC -4 CLASSIFICATION AND NOMENCLATURE OF ETHERS (LONG ANSWER TYPE QUESTION -II)|1 VideosALCOHOLS, PHENOLS AND ETHERS
OSWAAL PUBLICATION|Exercise TOPIC -4 CLASSIFICATION AND NOMENCLATURE OF ETHERS (VERY SHORT ANSWER TYPE QUESTIONS)|4 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
OSWAAL PUBLICATION|Exercise TOPIC -3 METHODS OF PREPARATION OF CARBOXYLIC ACID, PROPERTIES AND USES (LONG ANSWER TYPE QUESTIONS II)|7 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-ALCOHOLS, PHENOLS AND ETHERS-TOPIC -4 CLASSIFICATION AND NOMENCLATURE OF ETHERS (SHORT ANSWER TYPE QUESTIONS)
- Account for the following: (i) The boiling point of ethanol is highe...
Text Solution
|
- How would convert the following: (i) Phenol to benzoquinone (ii) P...
Text Solution
|
- Give the mechanism of preparation of ethoxy ethane from ethanol.
Text Solution
|
- Account for the following: (i) The boiling points of alchols decreas...
Text Solution
|
- Williamson's ether synthesis with an example.
Text Solution
|
- State the products of the following reactions: (i) CH(3)CH(2)CH(2)O...
Text Solution
|