Text Solution
Verified by Experts
Topper's Solved these Questions
ALDEHYDES, KETONES AND CARBOXYLIC ACIDS
OSWAAL PUBLICATION|Exercise TOPIC -2 METHODS OF PREPARATION OF ALDEHYDES AND KETONES (LONG ANSWER TYPE QUESTIONS -II)|1 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
OSWAAL PUBLICATION|Exercise TOPIC -3 METHODS OF PREPARATION OF CARBOXYLIC ACID, PROPERTIES AND USES (VERY SHORT ANSWER TYPE QUESTIONS)|4 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
OSWAAL PUBLICATION|Exercise TOPIC -2 METHODS OF PREPARATION OF ALDEHYDES AND KETONES (SHORT ANSWER TYPE QUESTIONS)|14 VideosALCOHOLS, PHENOLS AND ETHERS
OSWAAL PUBLICATION|Exercise TOPIC -4 CLASSIFICATION AND NOMENCLATURE OF ETHERS (LONG ANSWER TYPE QUESTION -II)|1 VideosBIOMOLECULES
OSWAAL PUBLICATION|Exercise TOPIC -II (LONG ANSWER TYPE QUESTIONS)|2 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-ALDEHYDES, KETONES AND CARBOXYLIC ACIDS-TOPIC -2 METHODS OF PREPARATION OF ALDEHYDES AND KETONES (LONG ANSWER TYPE QUESTIONS -I)
- a. Write the organic compound formed in the following equations: (...
Text Solution
|
- How do you convert benzoic acid to benzamide? Write the reaction.
Text Solution
|
- a. Explain the laboratory method of preparation fo p-bromoacetanilide ...
Text Solution
|
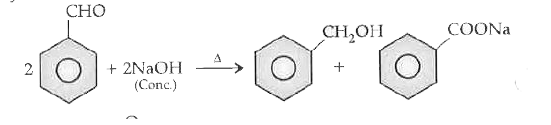
- How test benzaldehyde react with a concentrated solution of sodium hyd...
Text Solution
|
- Explain with equations how to convert: (i) Aldehyde containign no alp...
Text Solution
|
- How are following formed? (i) Cinnamic acid form benzaldehyde. (ii...
Text Solution
|
- How do carbonyl compounds react with HCN. Give mechanism of the reacti...
Text Solution
|