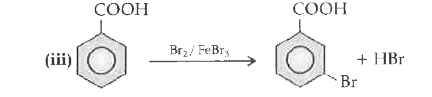Text Solution
Verified by Experts
Topper's Solved these Questions
ALDEHYDES, KETONES AND CARBOXYLIC ACIDS
OSWAAL PUBLICATION|Exercise TOPIC -3 METHODS OF PREPARATION OF CARBOXYLIC ACID, PROPERTIES AND USES (LONG ANSWER TYPE QUESTIONS I)|1 VideosALCOHOLS, PHENOLS AND ETHERS
OSWAAL PUBLICATION|Exercise TOPIC -4 CLASSIFICATION AND NOMENCLATURE OF ETHERS (LONG ANSWER TYPE QUESTION -II)|1 VideosBIOMOLECULES
OSWAAL PUBLICATION|Exercise TOPIC -II (LONG ANSWER TYPE QUESTIONS)|2 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-ALDEHYDES, KETONES AND CARBOXYLIC ACIDS-TOPIC -3 METHODS OF PREPARATION OF CARBOXYLIC ACID, PROPERTIES AND USES (LONG ANSWER TYPE QUESTIONS II)
- A ketone A(C(4)H(8)O) which undergoes a haloform reaction gives compou...
Text Solution
|
- a. Write the product of the followig reactions: (ii) 2C(6)H(5)CHO...
Text Solution
|
- a. Predict the products (i) CH(3)COCH(3)underset("Conc."HCl)overset(...
Text Solution
|
- a. Identify A,B and C in the following sequence of reactions: b. ...
Text Solution
|
- a. Describe the following giving linked chemical equations: (i) Cann...
Text Solution
|
- a. Give chemical tests to distinguish between the following: (i) Ben...
Text Solution
|
- Identify A to E in the following sequences of operations: CH(3)CH(2)...
Text Solution
|
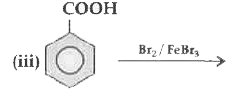
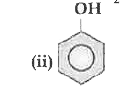 OR `CH_3 COOH`
OR `CH_3 COOH`