Similar Questions
Explore conceptually related problems
Recommended Questions
- An ideal gas at NTP is enclosed in an adiabatic vertical cylinder havi...
Text Solution
|
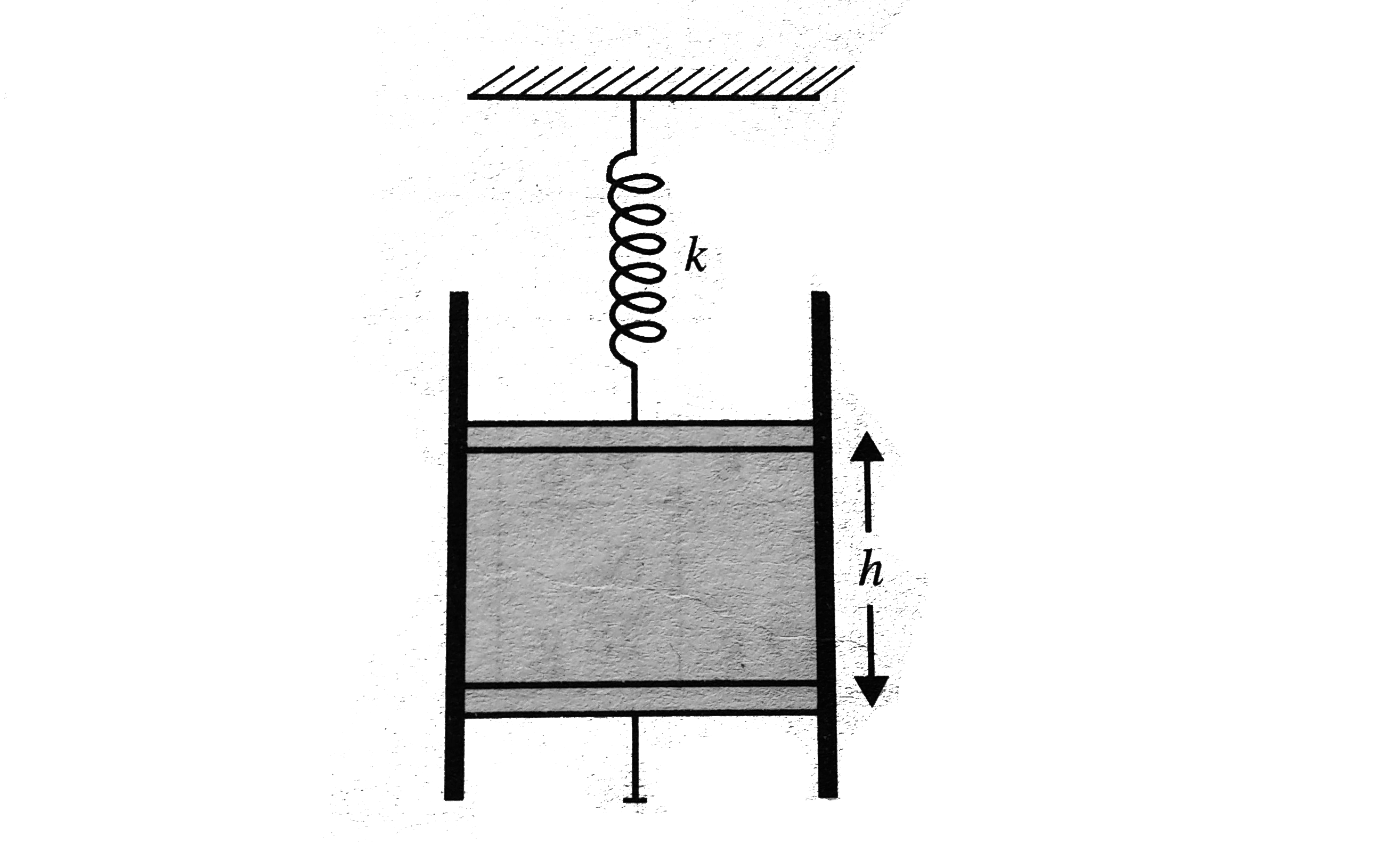
- An ideal monatonic gas is confined in a cylinder by a spring-loaded pi...
Text Solution
|
- 2.00 mol of a monatomic ideal gas (U=1.5nRT) is enclosed in an adiabat...
Text Solution
|
- A non conducting piston of mass m and area of cross section A is place...
Text Solution
|
- An ideal gas at NTP is enclosed in an adiabatic vertical cylinder havi...
Text Solution
|
- An ideal gas at NTP is enclosed in an adiabatic vertical cylinder havi...
Text Solution
|
- An ideal gas at NTP is enclosed in an adiabatic vertical cylinder havi...
Text Solution
|
- In ideal gas is enclosed in an adiabatic container having cross sectio...
Text Solution
|
- Two moles of an ideal monoatomic gas are confined within a cylinder by...
Text Solution
|