Similar Questions
Explore conceptually related problems
Recommended Questions
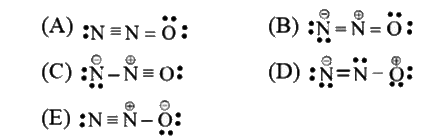
- N2O has a linear, unsymmetrical structure that may be thought of as a...
Text Solution
|
- Benzene is a resonance hybride of mainly two kekule structures. Hence
Text Solution
|
- The pair of structure that are resonance hybrid is:
Text Solution
|
- Amongst the given structures , which are permissible resonance forms ?
Text Solution
|
- Amongst the given structures , which are permissible resonance forms ?
Text Solution
|
- यद्यपि फीनॉक्साइड की पाँच अनुनादी संरचनाएँ होती हैं, किन्तु यह दो अनुन...
Text Solution
|
- Which of the following arrow is used between two structures to indicat...
Text Solution
|
- Aniline is resonance hybrid of structures
Text Solution
|
- कार्बोनेट आयन अनुनादी संरचनों का अनुनाद संकर है।
Text Solution
|