Similar Questions
Explore conceptually related problems
Recommended Questions
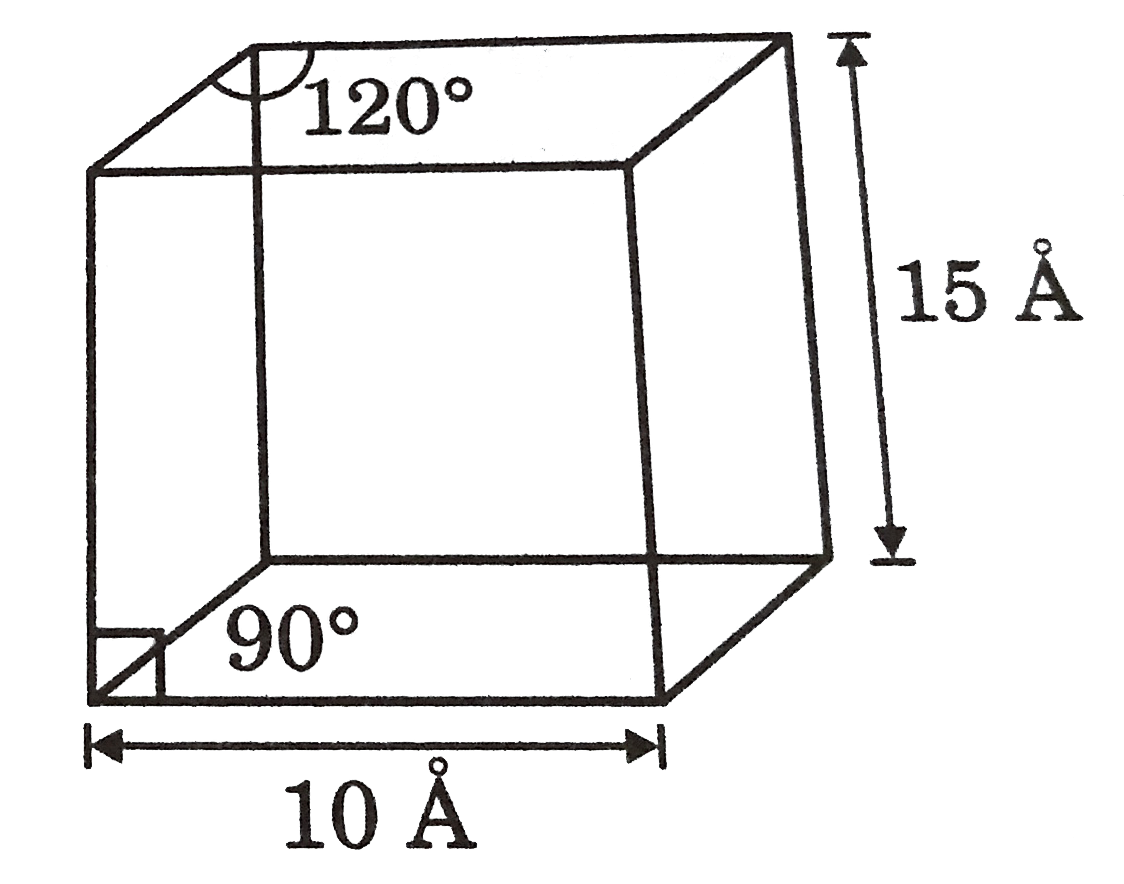
- A solid crystallines in a hexagonal structures as shown in the figure....
Text Solution
|
- Total number of atoms present in 1.0 cm^(3) of solid urea (density 0.3...
Text Solution
|
- A solid crystallines in a hexagonal structures as shown in the figure....
Text Solution
|
- A solid element (monoatomic) exists as cubic crystal. If its atomic ra...
Text Solution
|
- A solid AB has rock salt structure. How many atoms of A and B are pres...
Text Solution
|
- A binary solid, A^(+) B^(-) ( formula mass= 60) has a CsCl structure. ...
Text Solution
|
- An ionic solid PQ crystallises in rock salt structure with density 4.0...
Text Solution
|
- X=5Å Y=8Å Molar mass of solid ="259.8 g mol"^(-1) A solid crysta...
Text Solution
|
- A crystalline solid of pure substance has a face - centred cubic struc...
Text Solution
|