Similar Questions
Explore conceptually related problems
Recommended Questions
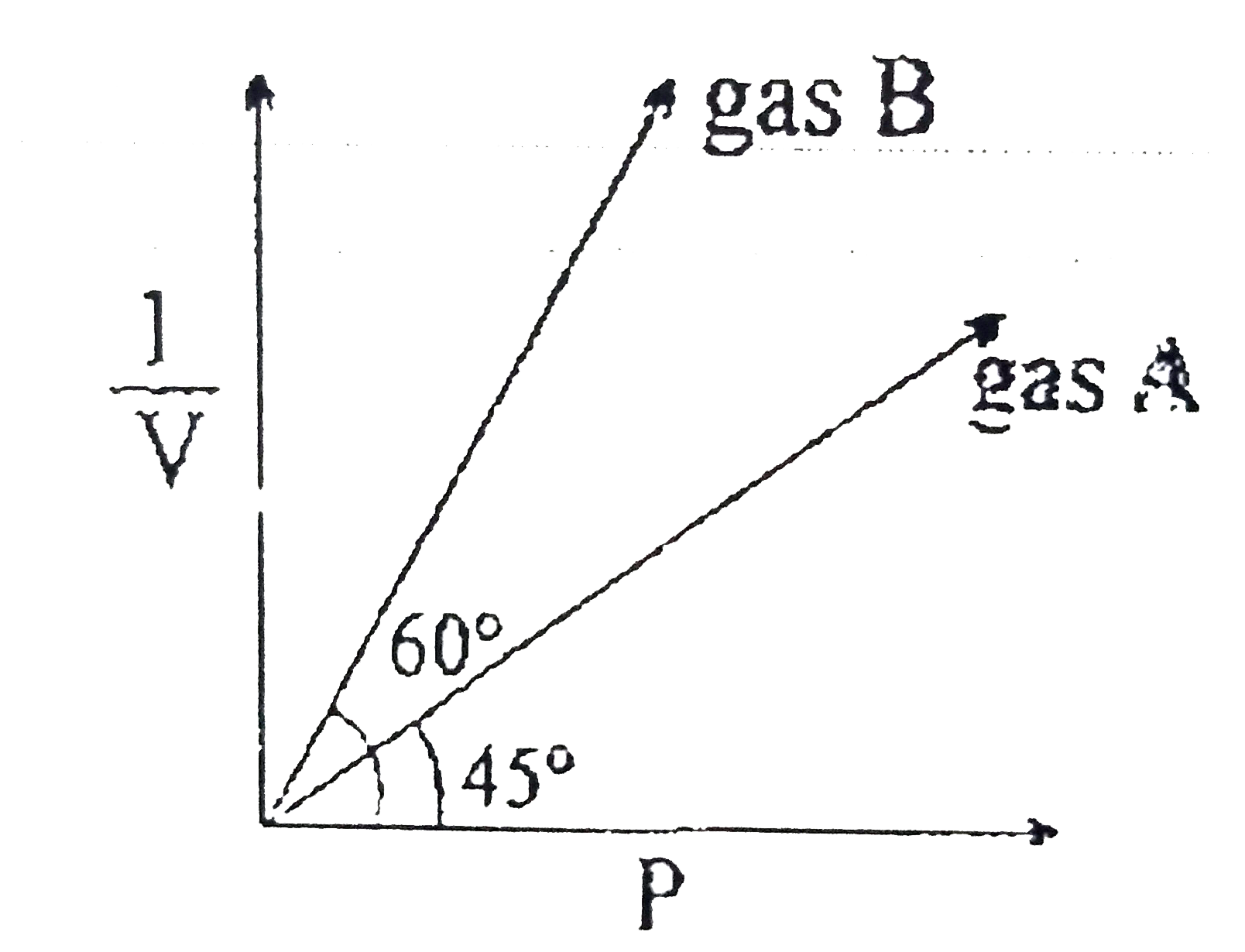
- At constant temperature of 273K. (1)/(v) vs are plotted for two ideal ...
Text Solution
|
- For one mole of a van der Waals' gas when b=0 and T=300K, the pV vs 1/...
Text Solution
|
- For 1 mole of an ideal gas, a graph of pressure vs volume is plotted a...
Text Solution
|
- For 1 mole of an ideal gas, a graph of pressure vs volume is plotted a...
Text Solution
|
- V vs T curves at constant pressure P(1) and P(2) for an ideal gas are ...
Text Solution
|
- For two samples of ideal gases A and B curves are plotted n vs V (volu...
Text Solution
|
- At constant temperature of 273 K, (1)/(V) vs P are plotted for two ide...
Text Solution
|
- At constant temperature of 273K. (1)/(v) vs are plotted for two ideal ...
Text Solution
|
- A plot of P vs T for a given mass of gas at constant volume is a strai...
Text Solution
|