Similar Questions
Explore conceptually related problems
Recommended Questions
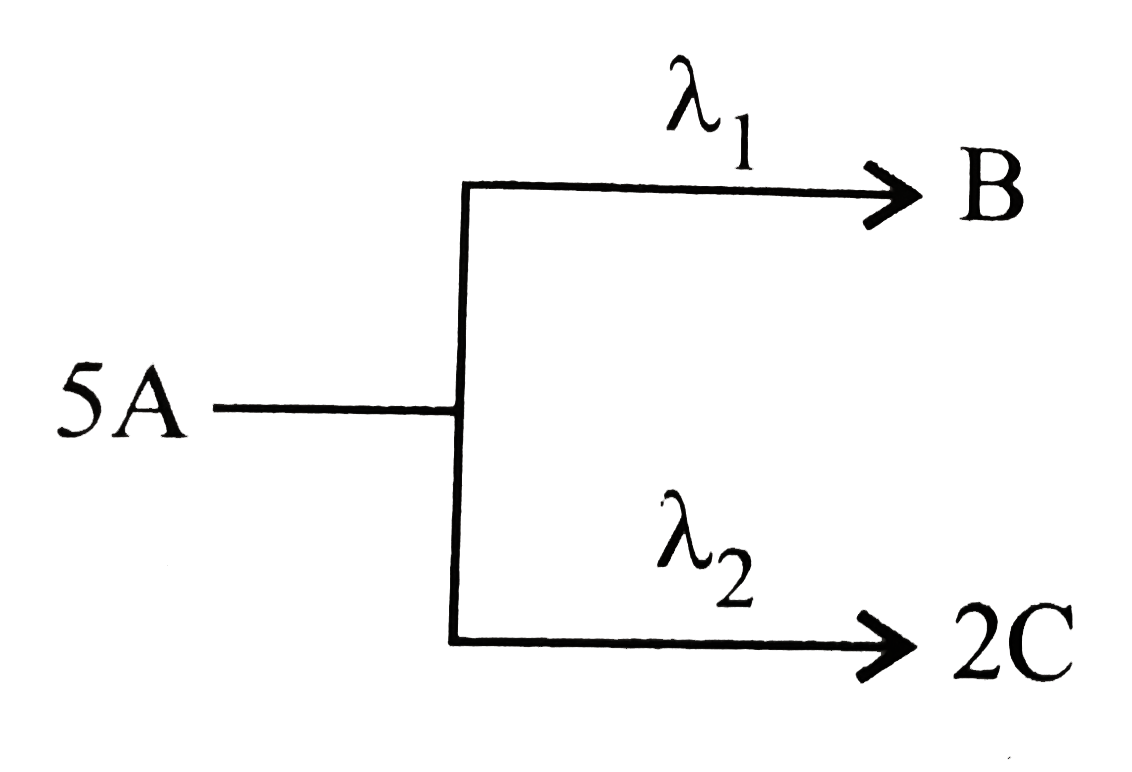
- A follow parallel path of first-order reactions giving B and C as If...
Text Solution
|
- The half-life periof and initial concentration for a reaction are as f...
Text Solution
|
- A follow parallel path of first-order reactions giving B and C as If t...
Text Solution
|
- A certain reaction A+B rarr C is first order with respect to each reac...
Text Solution
|
- For a first order reaction : ArarrB with initial concentration =a
Text Solution
|
- प्रथम कोटि की अभिक्रिया में, 40 सेकण्ड में पदार्थ की सान्द्रता प्रारम्...
Text Solution
|
- प्रथम कोटि की अभिक्रिया में, 72 सेकण्ड में पदार्थ की सान्द्रता प्रारम्...
Text Solution
|
- Reaction : A to B follows zero order kinetics and initial concentratio...
Text Solution
|
- A(g) to B(g) + C(g) Initial concentration of A is 20 M and concentrati...
Text Solution
|